In a new study recently published in Cell Reports Medicine, researchers from institutions including the College of Life Sciences at the University of Chinese Academy of Sciences, BGI-Research, and Tianjin Women and Children's Health Center have made advances in the early diagnosis and treatment of Gestational Diabetes Mellitus (GDM) through DNA analysis techniques.
 Longitudinal Integrative Cell-free DNA Analysis in Gestational Diabetes Mellitus published in Cell Reports Medicine
Longitudinal Integrative Cell-free DNA Analysis in Gestational Diabetes Mellitus published in Cell Reports Medicine
GDM, a condition characterized by high blood sugar levels that develop during pregnancy, affects roughly one in six pregnancies globally. It is not only a health concern for the mother, leading to an increased risk of type 2 diabetes and cardiovascular diseases later in life, but it also poses serious risks to the fetus, including growth abnormalities and premature birth. Despite significant strides in understanding and managing GDM, the underlying mechanisms of the disease remain elusive, making early diagnosis challenging and often delayed.
The study focused on the analysis of cell-free DNA (cfDNA) in maternal plasma. CfDNA consists of short fragments of DNA released primarily from cell apoptosis and has varying origins, including the placenta during pregnancy. Its analysis has traditionally been utilized in non-invasive prenatal testing to detect fetal chromosomal abnormalities but is now proving invaluable in identifying and understanding diseases like GDM.
Researchers undertook a nested case-control study analyzing cfDNA from 299 GDM patients and 299 healthy pregnant women across three trimesters, all of whom provided written informed consent. Their analysis revealed distinctive cfDNA fragment characteristics and dynamic changes tightly associated with GDM, including variations that signal anomalies in metabolic pathways.
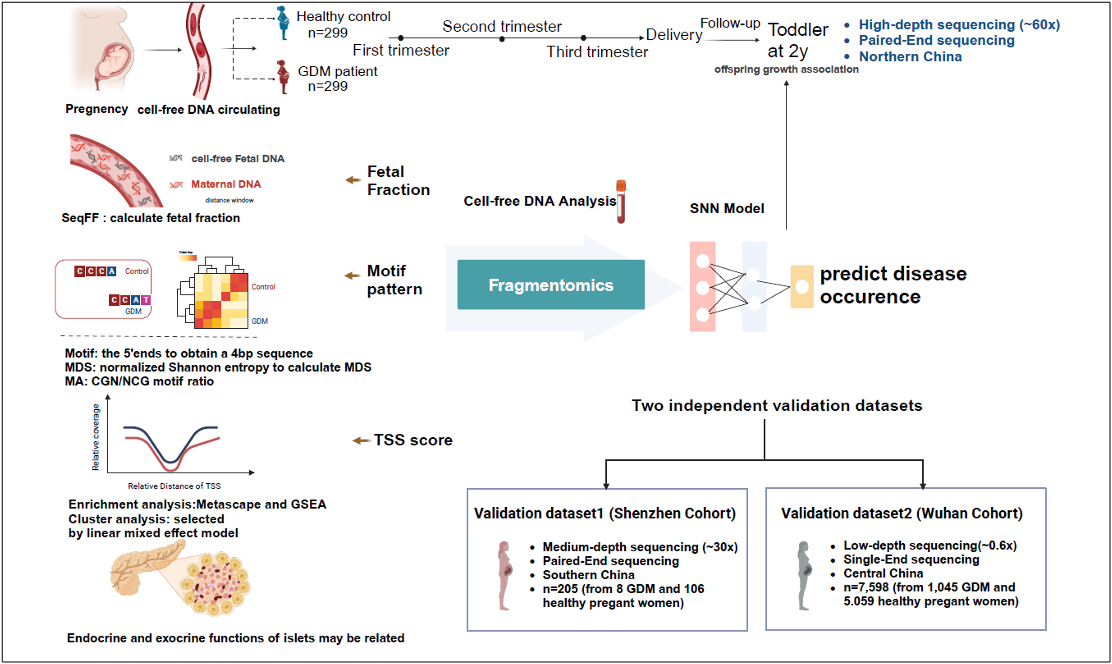 Study Design
Study Design
Additionally, the team successfully integrated lipidomics and single-cell transcriptomics data to delve deeper into the metabolic dysfunctions present in GDM patients. This comprehensive approach helped illuminate the functional variations in lipid metabolism and elucidated the typical biological pathways linked to GDM. Notably, specific cfDNA fragments from the PRSS1 gene showed a high correlation with GDM, pointing to a potentially valuable biomarker for early diagnosis.
Building on these insights, the research team developed a predictive model based on neural networks. This model assesses early-stage cfDNA characteristics such as the patterns of DNA sequences (motifs), the diversity of these motifs (MDS), and scores related to transcription start sites (TSS) to predict the onset of GDM. This model has been validated in two independent cohorts, confirming its efficacy and the universal applicability of these molecular features for predicting GDM across different populations.
Ethical review approval was obtained for this study.
The study can be accessed here: https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(24)00374-4



