The gut serves as the primary site for nutrient absorption and energy acquisition, and it facilitates symbiotic interactions between the host and its microbiota. Disruptions in this balance can lead to metabolic diseases like obesity. While much research has focused on beneficial bacteria and identified probiotics like Akkermansia muciniphila and Bacteroides thetaiotaomicron that enhance metabolism, studies on bacteria that contribute to obesity are still limited. It's not fully understood how specific obesity-linked bacteria influence obesity development. Additionally, genetics play a crucial role in obesity, with the interplay between genetics and the microbiome being a significant area of research interest.
A recent study conducted by a joint team from Ruijin Hospital,Shanghai Jiao Tong University School of Medicine and BGI-Research have significantly advanced our understanding of how gut microbiota is linked to obesity. Published in the journal Cell Host & Microbe, their study provides a groundbreaking look at how specific gut bacteria, particularly a species of Megamonas, contribute to obesity by enhancing lipid absorption through the degradation of myo-inositol.
 Obesity-enriched gut microbe degrades myo-inositol and promotes lipid absorption published in Cell Host & Microbe.
Obesity-enriched gut microbe degrades myo-inositol and promotes lipid absorption published in Cell Host & Microbe.
The study emerged from the recognition that while many studies have identified the gut microbiota's crucial role in obesity, the specific microbes and mechanisms involved remained largely unknown. Addressing this gap, the research team conducted shotgun metagenomic sequencing on a large cohort comprising 631 obese subjects and 374 normal-weight controls in China. They identified an enterotype-like cluster dominated by Megamonas, which was notably enriched in obese subjects and showed strong associations with obesity phenotypes.
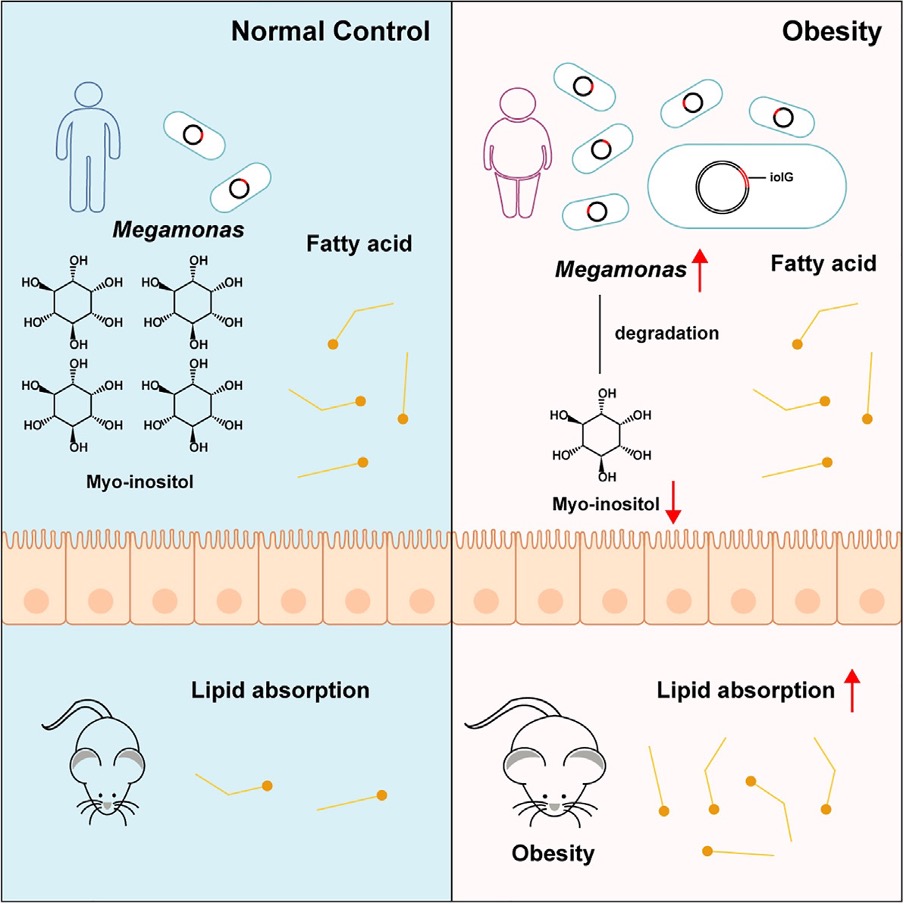 Graphical abstract of the study
Graphical abstract of the study
Among the findings, the presence of Megamonas and a polygenic risk score (PRS) —an aggregate measure of genetic susceptibility to obesity—exhibited an additive impact on the condition. Megamonas rupellensis, the species under scrutiny, was found to possess genes capable of degrading myo-inositol, a type of sugar alcohol involved in cellular signalling and fat metabolism. Both in vitro and in vivo experiments confirmed the bacterium’s ability to degrade myo-inositol, leading to increased fatty acid absorption in the intestines.
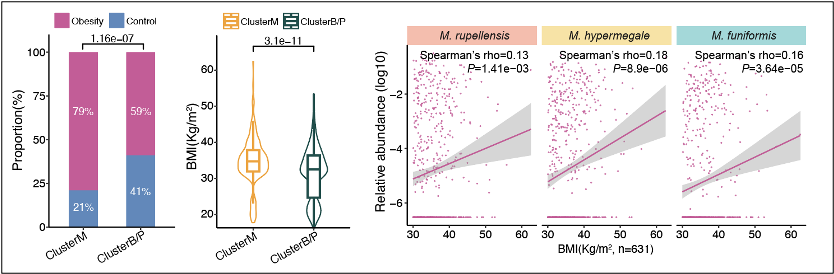 Megamonas (ClusterM and Megamonas species) are positively correlated with obesity.
Megamonas (ClusterM and Megamonas species) are positively correlated with obesity.
Further experiments reinforced these findings. When intestinal organoids were exposed to myo-inositol, fatty acid absorption was effectively inhibited, suggesting a direct pathway through which this gut microbe influences obesity. Moreover, the researchers introduced the myo-inositol-degrading gene iolG from Megamonas into E. coli bacteria. Mice colonized with either M. rupellensis or the genetically modified E. coli exhibited increased intestinal lipid absorption, resulting in significant weight gain and fat accumulation, particularly when fed a high-fat diet.
These results are particularly striking as they suggest a mechanistic link between a specific microbial pathway and obesity, mediated through the degradation of myo-inositol and enhanced lipid absorption. This finding paves the way for new strategies in obesity management, potentially through altering the gut microbiota. By targeting the metabolic pathways associated with specific bacteria like Megamonas, it may be possible to develop therapeutic strategies that can either inhibit or enhance specific microbial actions to manage or prevent obesity.
The research also highlighted the influence of genetic factors on the effectiveness of gut microbiota in influencing obesity. Individuals with a lower genetic risk score showed a greater variance in BMI due to differences in their gut microbiota compared to those with higher genetic predispositions. This finding underscores the complex interplay between genetics and microbiota, emphasizing the need for personalized approaches in treating and managing obesity.
Looking forward, the team plans to further explore the therapeutic potentials of targeting myo-inositol degradation in the gut. Such strategies could involve the development of microbiota-targeted therapies that either block harmful bacterial actions or promote beneficial ones, thereby adjusting the body's lipid metabolism to combat obesity.
Ethical review approval was obtained for this study.
The study can be accessed here: https://doi.org/10.1016/j.chom.2024.06.012



