The mysteries of how we age have been studied by scientists for centuries. In a landmark study published in Cell on November 4, researchers produced the world’s first spatiotemporal atlas of multi-organ aging in male mouse. This atlas provides a deeper understanding of how aging affects gene expression and cell interactions within the tissue microenvironment, and highlighting the potential for interventions aimed at promoting healthy aging.
 The study “Spatial Transcriptomic Landscape Unveils Immunoglobin-associated Senescence as a Hallmark of Aging”was published in Cell.
The study “Spatial Transcriptomic Landscape Unveils Immunoglobin-associated Senescence as a Hallmark of Aging”was published in Cell.
The study, “Spatial Transcriptomic Landscape Unveils Immunoglobin-associated Senescence as a Hallmark of Aging,” was co-led by BGI-Research, the Institute of Zoology at the Chinese Academy of Sciences, and the Beijing Institute of Genomics at the Chinese Academy of Sciences, and also involved scientists from other institutions.
The billions of cells in living organisms work closely together to maintain life activities, however, in aging tissues, molecular and cellular changes disrupt the spatial organization, resulting in uneven functional decline. Scientific understanding of how aging triggers tissue and cell degeneration at the spatial level is still limited, and revealing the core drivers of aging in the complex spatiotemporal context is an important challenge for the scientific research of aging.
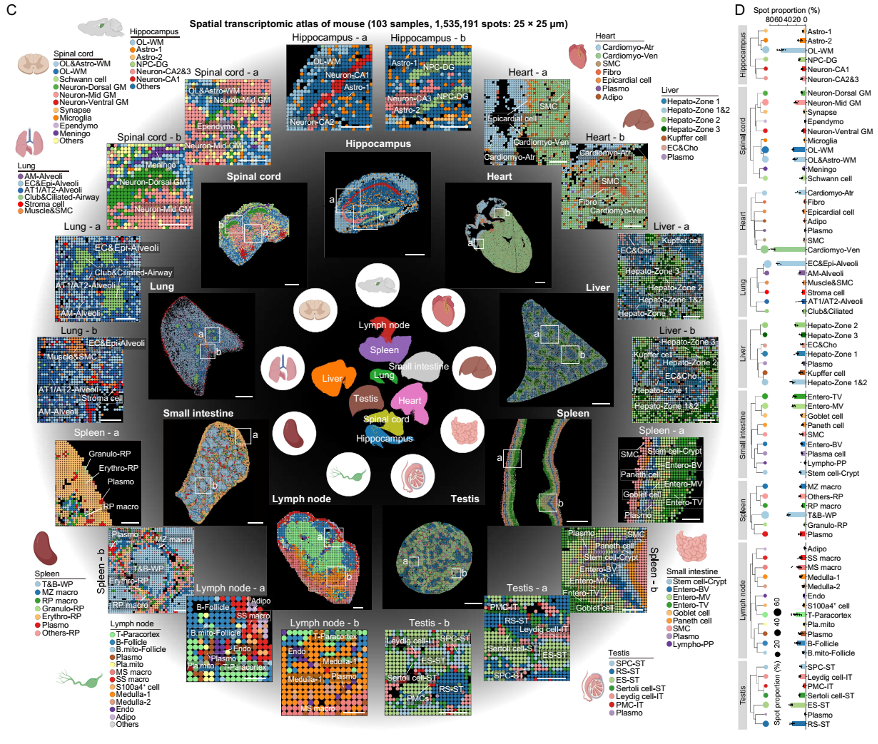 Spatial transcriptomic atlas of young mouse tissues.
Spatial transcriptomic atlas of young mouse tissues.
To systematically characterize the loss of tissue integrity and organ dysfunction resulting from aging, the team used Stereo-seq, BGI’s independently-developed spatial transcriptomic technology, to construct a comprehensive spatiotemporal map of multi-organ aging by performing high resolution analysis of gene expression in nine tissues of fully developed male mice at different life stages from two months to 25 months (equivalent to more than 80 human years). The multi-organ spatiotemporal map covers the hippocampus, spinal cord, liver, spleen, heart, lung, small intestine, testicle, and lymph nodes, and contains 72 cell types and more than two million spatiotemporal points.
This map enables researchers to gain deeper insights into how cells maintain their organizational structure and function, the aging processes of cells and organs, and the impact of immunoglobins (proteins that help the body fight infection) on aging. The study not only pinpointed the core regions of aging in multiple organs, but also found that the accumulation of immunoglobulins is a key feature and driver of aging, which shows that aging is not a straightforward process; instead, it involves specific areas in different organs that age at different rates. This discovery provides a new scientific basis for a deeper understanding of the mechanisms, early warnings, and interventions of aging.
In earlier studies it had been shown that Organizational Structure Entropy (OSE) increases with age. In this study, researchers found that the most pronounced increase in entropy occurred in the hippocampus, spleen, lymph nodes, and liver. They also discovered that an increase in entropy is associated with a loss of cellular identity, suggesting that structural disruption can lead to functional decline in senescent tissues, resulting in chronic inflammation and age-related dysfunction.
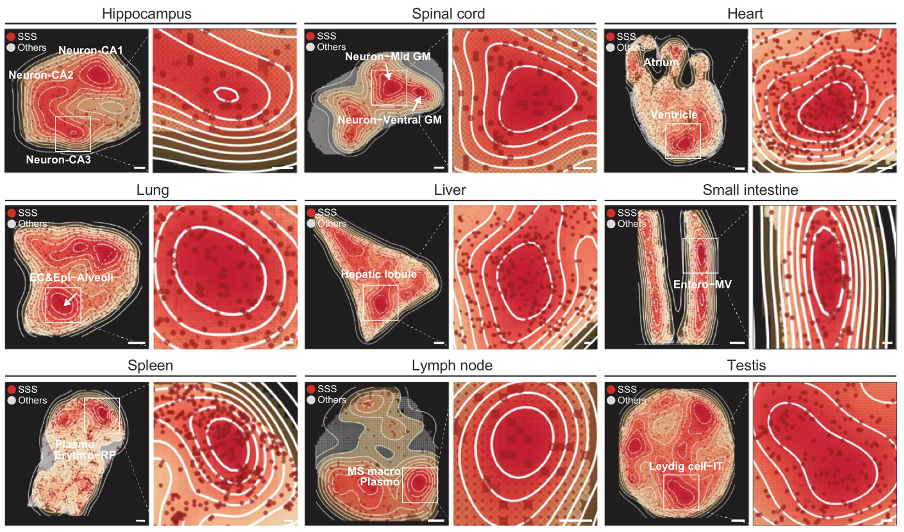 Spatial mappings showing the density areas of SSSs
Spatial mappings showing the density areas of SSSs
The research team then constructed a specific set of sensitive genes for the spatial location of aging, and identified a key senescence-sensitive site, termed Senescence-Sensitive Spots (SSS). It was found that the increase in tissue entropy and the loss of cell identity were more pronounced near the SSS region. Additionally, in immune organs, plasma cells, which produce antibodies to help fight infections, along with other specialized cells, are key components of the area around senescence-sensitive spots (SSS). The study found that the closer these cells are to the SSS, the higher the expression levels of genes related to immunoglobulins.
During aging, the levels of immunoglobulin G (IgG) often increase, and their accumulation in various tissues and organs may serve as a new indicator of aging. Research has demonstrated that high IgG levels can induce aging in immune cells, like macrophages and microglia, leading to greater inflammation in both humans and mice. By studying how IgG affects the aging process, researchers aim to develop methods to further slow aging, minimize visible signs of aging, and reduce IgG levels to the normal range found in younger individuals, even though IgG levels typically rise with age.
Aging is a complex biological process with organs aging at different rates. However, by employing the latest technologies, such as Stereo-seq, scientists are able to add to the body of research about the aging process.
With the deepening of research, the link between immunoglobulins and human aging will lead to more scientific explorations, such as: the cellular origin of immunoglobulins in the aging process; the regulatory mechanism at the genome level; whether they produce targeted neutralizing activity against autoantigens; whether human biological age can be evaluated based on the accumulation of immunoglobulins; whether they have the potential to become markers and drivers of aging-related diseases; whether they can intervene in aging and related diseases by targeting IgG or its downstream pathways; and, whether antibody-based immunotherapies may accelerate aging or degeneration of the body due to overuse. It is expected that technological advances will gradually reveal these puzzles. Bringing us closer than ever to understanding how to live longer and better.
Ethical review approval was obtained for this study.
This study can be accessed here: https://www.cell.com/cell/abstract/S0092-8674(24)01201-7



