New research by BGI-Research and Wuhan University used single-cell multi-omics sequencing to reveal the adaptive changes of Glioblastoma (GBM) cells and provides a direction for a possible dual-target precision treatment strategy. The research is published on November 22 by Science Advances as “Single-cell multi-omics sequencing uncovers region-specific plasticity of glioblastoma for complementary therapeutic targeting.”
 The Study “Single-cell multi-omics sequencing uncovers region-specific plasticity of glioblastoma for complementary therapeutic targeting” was published in Science Advances.
The Study “Single-cell multi-omics sequencing uncovers region-specific plasticity of glioblastoma for complementary therapeutic targeting” was published in Science Advances.
GBM is the most aggressive and common type of brain cancer and grows rapidly. The high heterogeneity and aggressiveness of GBM cells make it difficult to be completely eliminated through treatment. While conventional treatments such as surgical resection, radiotherapy, and chemotherapy are widely available, tumor recurrence is very high and the median survival of patients is only 15 months.
The heterogeneity of GBM is manifested in the dynamic transformation of tumor cells between several different states, including neural-progenitor-like (NPC-like), oligodendrocyte-progenitor-like (OPC-like), astrocyte-like (AC-like), and mesenchymal-like (MES-like). These cellular states co-exist within the tumor, but can also switch to each other under therapeutic stress, thereby building drug resistance.
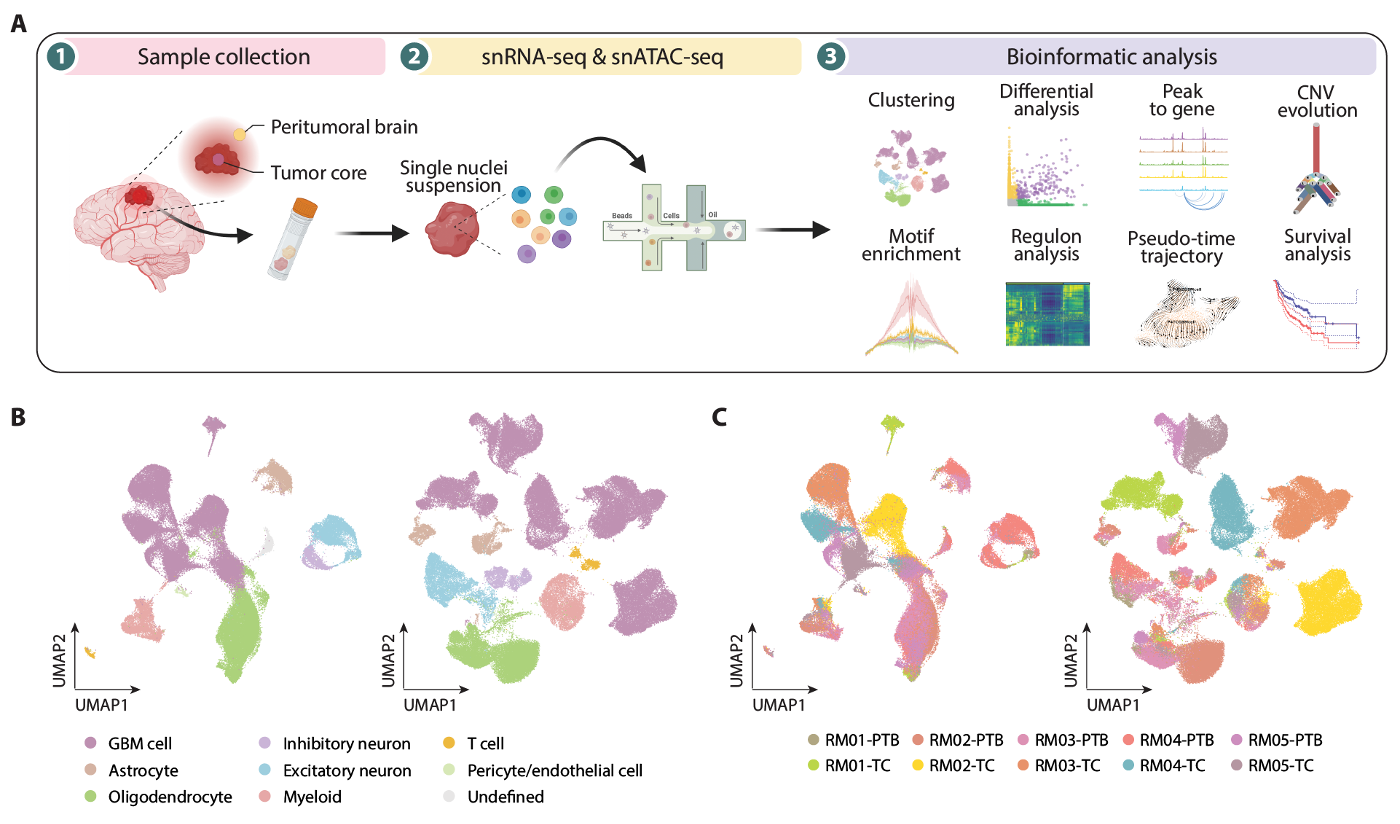 Single-cell atlas of gene expression and chromatin accessibility in glioblastoma.
Single-cell atlas of gene expression and chromatin accessibility in glioblastoma.
Also, invasiveness causes GBM cells to spread to normal brain tissue forming diffuse infiltrates, and these infiltrations remain alive and latent after treatment, waiting for tumor reoccurrence under suitable conditions. The small number of tumor cells in the area surrounding the tumor and the mixing with a large number of normal cells make comprehensive characterization of these tumor cells difficult.
The research team collected tissue samples from tumor regions and peritumoral brain regions of GBM patients, and used single-cell sequencing to analyze the gene expression and chromatin accessibility of tumor cells.
The data revealed significant molecular differences between the two regions of tumor cells in the GBM, with a significantly higher proportion of OPC-like cells in the peritumoral brain region, while the proportion of AC-like cells decreased. This suggests that malignant cells may acquire proneural features by establishing contact with local non-malignant cells, or that cells with a higher proneural state may spread easier.
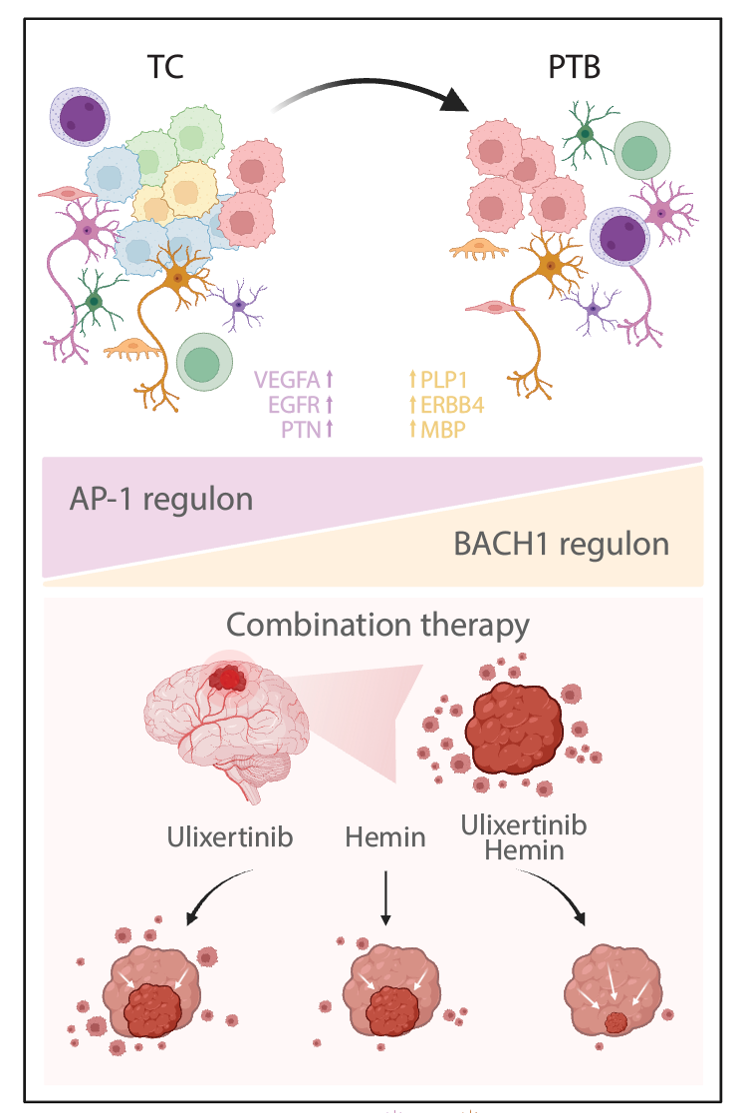 Combination Targeted Therapy for Glioblastoma.
Combination Targeted Therapy for Glioblastoma.
The researchers also identified that while activating protein 1 (AP-1) is active in all GBM states, its activity declines from the tumor region to the peritumoral region, while another transcription factor, BACH1, demonstrated the opposite trend.
Through further functional validation experiments, the researchers determined that a combination of targeting AP-1 and BACH1 transcription factors can effectively combat the heterogeneity and infiltration capability of GBM, providing a possible dual-target precision treatment strategy. In the future, this strategy could provide a more effective weapon for clinical treatment, especially in the treatment of GBM.
Ethical review approval was obtained for this study.
This study can be accessed here: https://www.science.org/doi/10.1126/sciadv.adn4306



