On January 1, 2025, a research team led by BGI-Research published a groundbreaking study in Cell Reports titled, “Multi-omics reveals immune response and metabolic profiles during high-altitude mountaineering”. The scientists applied single-cell transcriptomics and advanced mass spectrometry to profile over 375,000 immune cells and hundreds of plasma metabolites from mountaineers. The results add to the scientific understanding of how the human body’s immune system and metabolism change and adapt during high-altitude mountaineering.
 The study “Multi-omics reveals immune response and metabolic profiles during high-altitude mountaineering” was published in Cell Reports.
The study “Multi-omics reveals immune response and metabolic profiles during high-altitude mountaineering” was published in Cell Reports.
High-altitude mountaineering is an extreme sport, presenting climbers with low-pressure hypoxia, extreme cold, low humidity, and intense UV radiation. While the body compensates for reduced oxygen by boosting ventilation, cardiac output, and tissue oxygen uptake, extended physical exertion at these altitudes often increases physiological and metabolic stress—potentially leading to ailments like acute mountain sickness, weight loss, immune dysfunction, and heightened infection risks.
By collecting samples at five key points from 11 climbers during the ascent of a 7,546-meter-high mountain, the research team employed single-cell sequencing to map 375,722 circulating immune cells and used targeted mass spectrometry to quantify 309 metabolites and 717 complex lipids in plasma. Their analyses traced the immune and metabolic shifts during the acclimatization phase and the phase of extreme-altitude ascent.
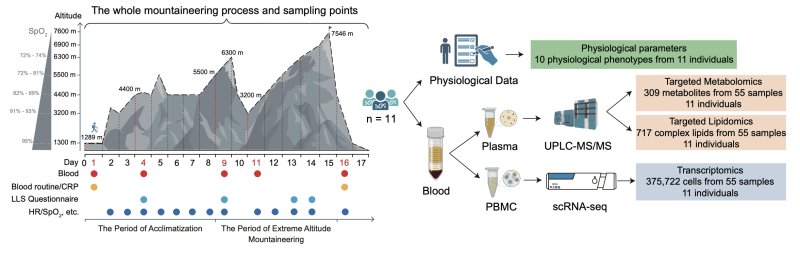 Research Design
Research Design
A high-resolution single-cell atlas of the climbers’ peripheral immune cells showed that the composition of different immune cell types changed substantially throughout the climb. Further sub-clustering of the main immune subsets highlighted dynamic differences between the acclimatization phase—when inflammatory responses in some myeloid cells were suppressed but cytotoxic CD8+ T cells, γδT cells, and CD16+ natural killer (NK) cells were more active—and the extreme-altitude stage, which saw heightened inflammation but reduced T-cell effector function. During this extreme exposure, immune cells also displayed pronounced responses to hypoxia and oxidative stress.
Particularly noteworthy was the upregulation of glycolysis and antioxidant genes, including the transcription factors HIF1A and NFE2L2, in several immune cell populations during high-altitude climbing. These findings indicate that immune cells undergo metabolic reprogramming to boost antioxidant defenses—essential for maintaining immune functions in severely hypoxic environments. Metabolite profiling also showed significant changes in glutamine and fatty acid pathways in the blood, suggesting enhanced energy metabolism under such conditions.
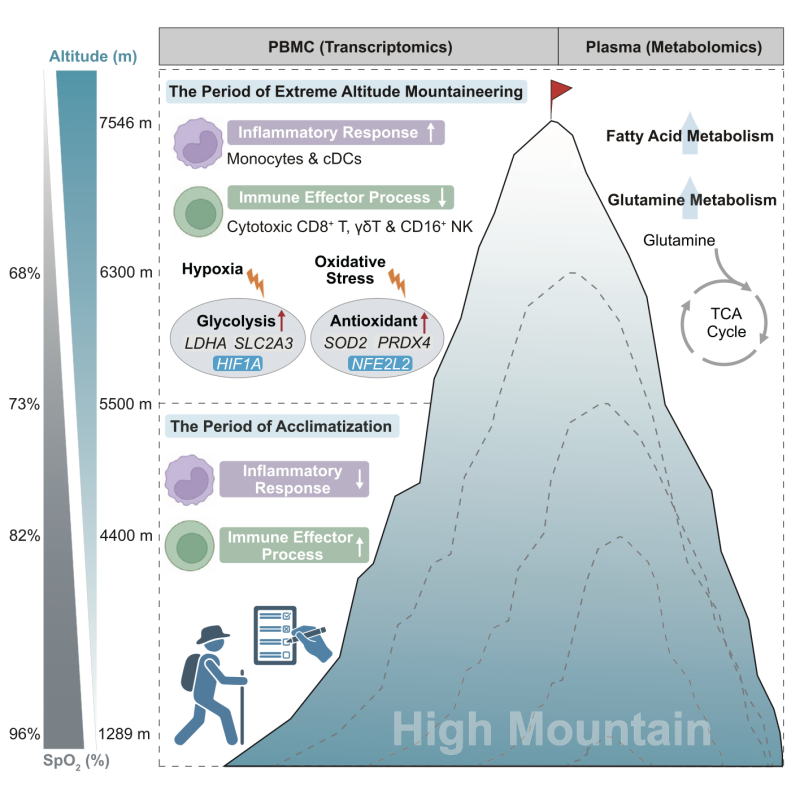 Graphical Abstract of the Publication
Graphical Abstract of the Publication
This study is part of “BGI Qomolangma Project” launched in 2022 with an ambitious goal to ascend several high-altitude peaks—including Mount Qomolangma (Mount Everest) —by 2024. Throughout these expeditions, volunteers provide biological samples and data, allowing researchers to investigate the mechanisms of human adaptation through cutting-edge multi-omics methods. Taken together, these multi-omics insights illuminate how the body orchestrates complex immunological and metabolic adaptations to withstand the challenges of high-altitude ascent.
Future research will focus on even higher elevations, including the summit of Qomolangma, to further unravel the mechanisms behind human adaptation.
The research protocol received approval from BGI’s institutional review board of bioethics and biosafety, and written informed consent was obtained from each individual participating in the mountaineering expedition.
The research paper can be assessed here: https://www.cell.com/cell-reports/pdf/S2211-1247(24)01485-2.pdf



