On February 10, scientists from Huazhong University of Science and Technology and BGI-Research have uncovered the intricate immune mechanisms underlying Fulminant Myocarditis (FM). Utilizing BGI’s Stereo-seq multi-omics technology alongside single-nucleus RNA sequencing (snRNA-seq), the study provides an unprecedented spatiotemporal perspective on FM, reshaping global understanding of this life-threatening cardiac condition. This study was published in Signal Transduction and Targeted Therapy.
 The study Spatiotemporal transcriptomics elucidates the pathogenesis of fulminant viral myocarditis was published in Signal Transduction and Targeted Therapy.
The study Spatiotemporal transcriptomics elucidates the pathogenesis of fulminant viral myocarditis was published in Signal Transduction and Targeted Therapy.
FM is the most severe form of acute myocarditis (AM), an inflammatory disease of the myocardium often triggered by viral infections or autoimmune responses. This condition predominantly affects children and young adults under 40, with an alarming in-hospital mortality or heart transplantation rate of over 25% and a five-year survival rate below 50%. Despite previous studies linking FM progression to immune system overactivation, the exact molecular and cellular mechanisms have remained unclear due to the sudden onset, focal inflammatory lesions, and the complex interplay of immune cells and viral interactions in cardiac tissues.
To address these knowledge gaps, researchers employed single-nucleus transcriptomics and spatial transcriptomics to analyze virus-induced FM mouse models. They discovered that mesothelial cells are the primary targets of viral infection, triggering a cascade of severe inflammatory responses leading to tissue damage. As infection advances, an increasing number of cardiomyocytes (heart contraction muscle cells) succumb to necrosis. Concurrently, immune cells, including macrophages and CD8+ effector T cells, proliferate at an accelerated rate.
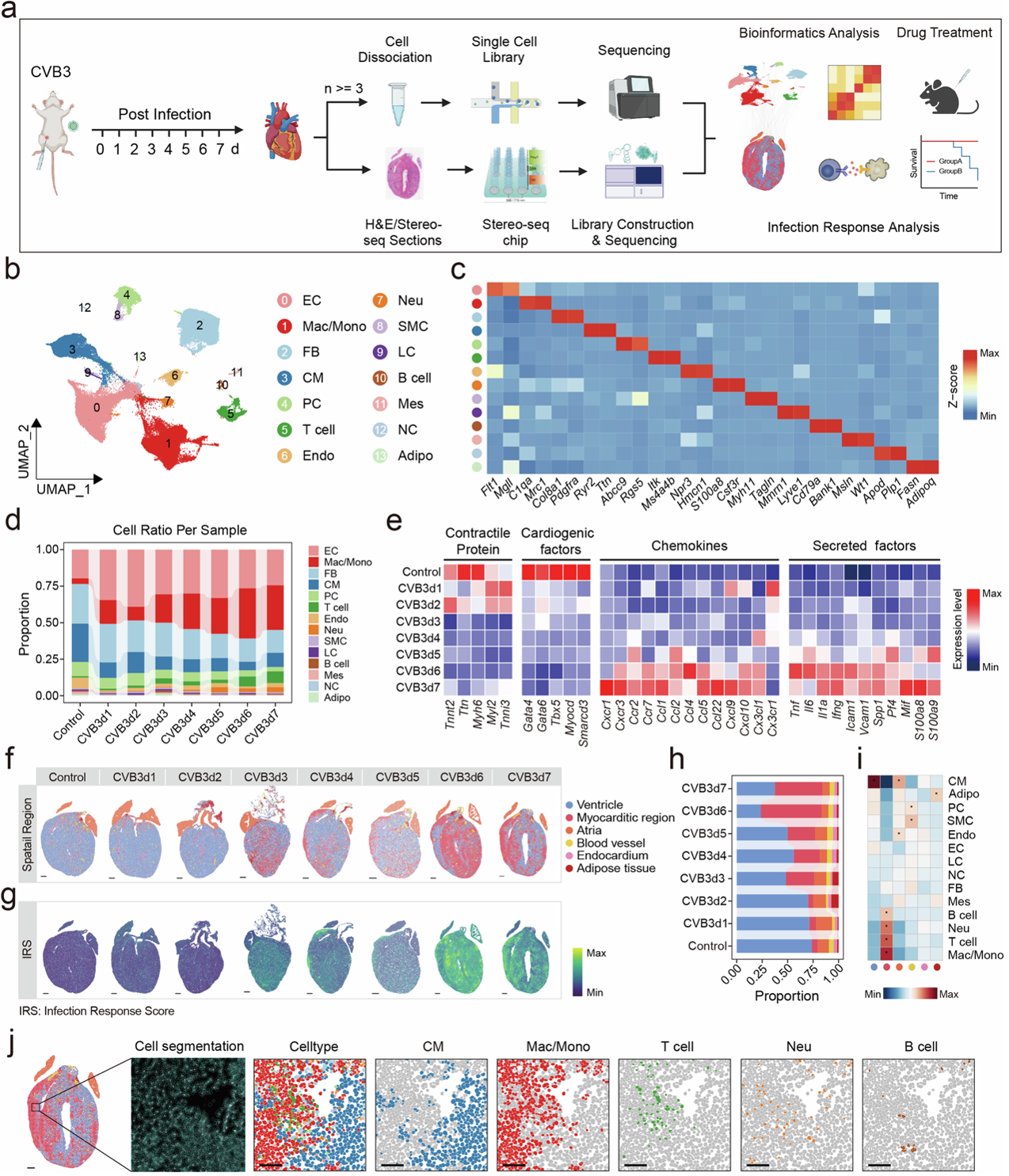 The spatiotemporal landscape of FM mice.
The spatiotemporal landscape of FM mice.
Notably, the study found that not all cardiomyocyte death is a direct result of viral infection. Spatial transcriptomic analysis revealed that immune cells’ cytotoxic activity significantly suppresses cardiac contraction gene expression. These immune cells were found very close to non-infected, yet dying, cardiomyocytes, suggesting that immune-mediated damage plays a crucial role in FM progression.
A key discovery was the role of interferon-gamma (IFN-γ), a cytotoxic cytokine released by immune cells, in exacerbating myocardial damage. Researchers identified that IFN-γ upregulates the expression of Spi1 gene, thereby amplifying cardiomyocyte death and further accelerating disease progression. This novel "IFN-γ / Spi1 axis" was found to be a critical driver of FM pathology.
To validate this finding, researchers administered Spi1 inhibitors to FM-afflicted mice, leading to remarkable improvements. The mortality rate significantly dropped, immune cell infiltration decreased, and heart function showed notable recovery. These findings suggest that targeting the IFN-γ / Spi1 axis could serve as a promising therapeutic strategy for FM.
Given the pivotal role of overactive immune responses in FM progression, researchers also explored the therapeutic effects of intravenous immunoglobulin (IVIG) - a well-established immunomodulatory treatment – and the results were striking. IVIG administration significantly reduced inflammation and viral RNA presence. The number of inflammatory macrophages and CD8+ effector T cells drastically declined, and mortality rates in FM mice plummeted from nearly 70% to 0%. These findings underscore IVIG's potent immunomodulatory effects, highlighting its potential as a life-saving therapy for FM patients.
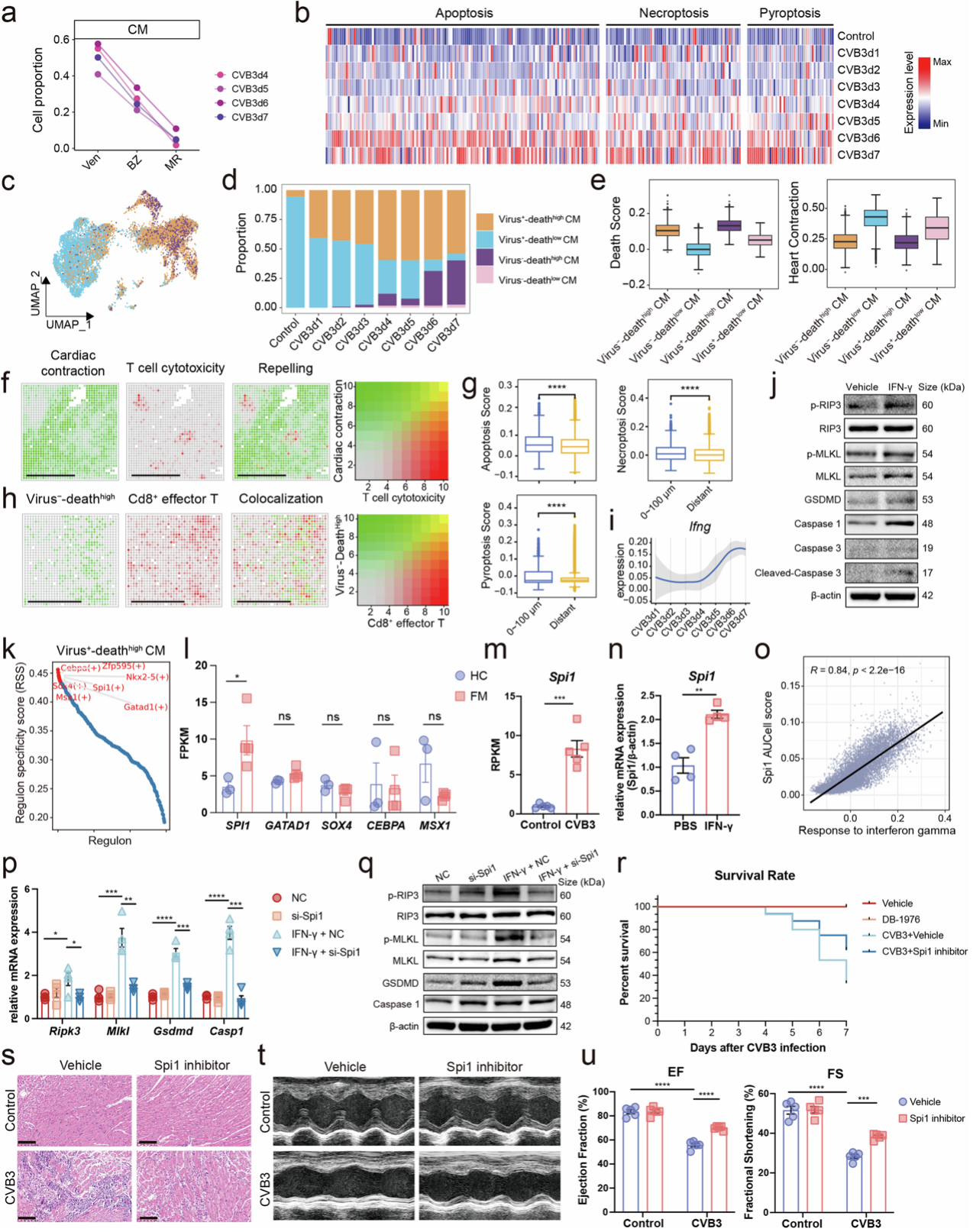 Cd8+ effector T cells induced the damage of cardiomyocytes.
Cd8+ effector T cells induced the damage of cardiomyocytes.
Reflecting on the significance of the study, Dr. Xiaodong Fang, co-corresponding author of the study and a researcher from BGI-Research stated: “This research establishes a comprehensive spatiotemporal landscape of FM, providing unique insights into its pathophysiology. By integrating single-cell sequencing and spatial transcriptomics, we have identified previously unknown functional pathways and spatial immune interactions. These discoveries enhance our understanding of FM’s pathogenic mechanisms and lay the foundation for novel therapeutic strategies.”
With these groundbreaking findings, the study paves the way for targeted treatments that could drastically improve survival rates and long-term outcomes for FM patients worldwide. As researchers continue to refine these therapeutic approaches, the hope of more effective clinical interventions for fulminant myocarditis grows stronger.
Ethical approval for this study was obtained.
The study can be accessed here: https://www.nature.com/articles/s41392-025-02143-9



