For more than a century, scientists have marveled at the striking disparities in regenerative ability across mammalian species. A rabbit can mend a hole punched clean through its outer ear in about a month, followed by the complete regeneration of internal structures (including cartilage, blood vessels and nerves, etc.) with scarcely a scar in approximately three months. A laboratory mouse presented with the identical wound in the same location fails to regenerate completely.
In a paper now published in Science, investigators from BGI-Research, the National Institute of Biological Sciences, Beijing (NIBS) and other institutes combine single-cell sequencing, BGI's high-resolution Stereo-seq spatial transcriptomics and 3-D chromatin mapping to explain that difference and, crucially, to remodel it.
 BGI-Research and the National Institute of Biological Sciences trace the “missing retinoic-acid switch” that throttles tissue regeneration in higher mammals, published in Science.
BGI-Research and the National Institute of Biological Sciences trace the “missing retinoic-acid switch” that throttles tissue regeneration in higher mammals, published in Science.
By comparing five mammalian species the team shows that mice and rats have, over evolutionary time, lost several DNA "remote controls" that normally flick on the gene Aldh1a2, the rate-limiting enzyme that converts vitamin A into the morphogen retinoic acid (RA). Without that burst of RA, the blastema (the mound of stem-like cells that appears after injury) receives the wrong biochemical instructions, stalls and leaves the ear permanently perforated.
When the researchers re-installed Aldh1a2 with an integrating AAV vector, supplied RA by low-dose injection, or transplanted a single rabbit enhancer into the mouse genome, the wound closed, cartilage re-materialised and sensory nerves grew back: in effect, the non-regenerating mouse was pushed toward the rabbit's regenerative programme.
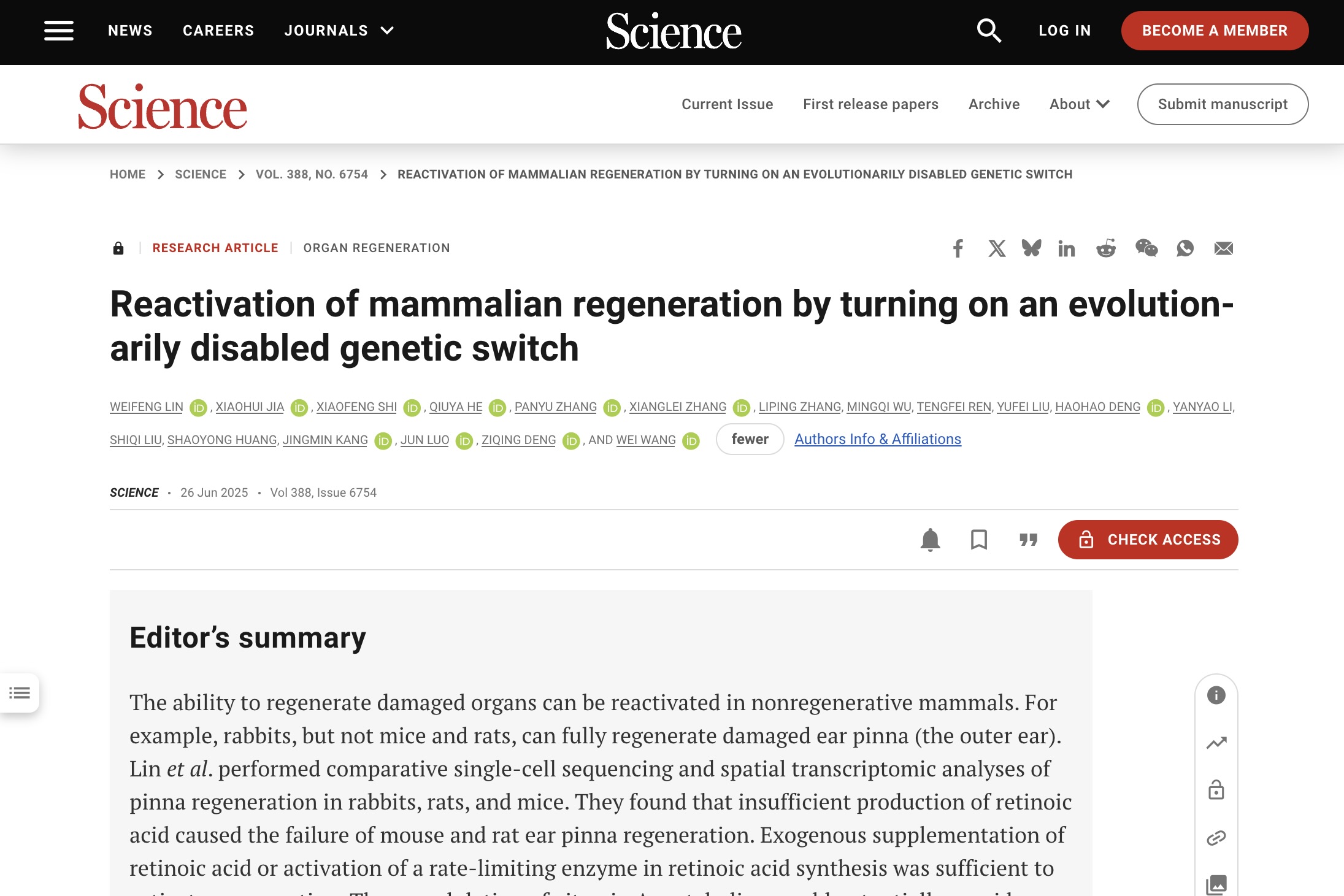
This discovery rests on the cell-by-cell, pixel-by-pixel maps generated with BGI's Stereo-seq chips. Those maps highlighted a specialised population dubbed wound-induced fibroblasts (WIFs), which are present in both rabbits and mice but transcriptionally primed for growth and morphogenesis only in the regenerative species. Stereo-seq also revealed that the WIF micro-environment in mice is laced with cytokines that promote scarring, whereas in rabbits it is enriched in bone-morphogenetic ligands such as BMP2.
Upstream of those differences, chromatin immunoprecipitation and Micro-C uncovered six active enhancers at the rabbit Aldh1a2 locus, two of which ignite specifically after injury; their orthologous regions in mouse and rat sit silent. Knocking a single rabbit enhancer (AE1) into the safe-harbour Rosa26 locus of mice was enough to boost endogenous Aldh1a2 expression and shrink the ear hole, proving that regulatory decay rather than coding-gene mutation is the proximate cause of lost regeneration.
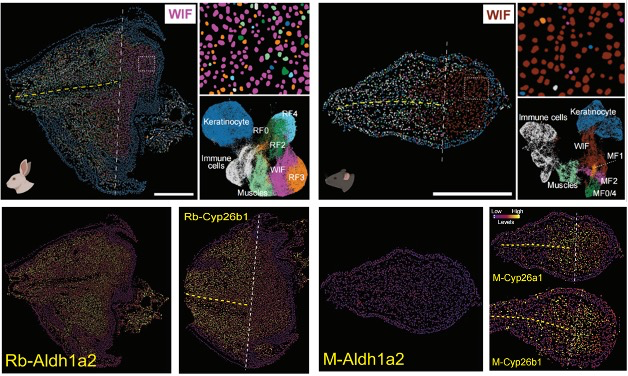 BGI's Stereo-seq maps show distinct wound-induced fibroblast responses between regenerating rabbit and repairing mouse ear tissue. This reveals the cellular basis for why evolutionary regenerative capacity was lost in rodents.
BGI's Stereo-seq maps show distinct wound-induced fibroblast responses between regenerating rabbit and repairing mouse ear tissue. This reveals the cellular basis for why evolutionary regenerative capacity was lost in rodents.
At the heart of this molecular switch lies retinoic acid, a derivative of vitamin A that acts as a master regulator of cellular fate during development and tissue repair. Systemic injections of retinoic acid at clinically relevant doses completely healed 2-mm ear defects in both mice and rats within 30 days, regenerating not just skin but also cartilage and sensory innervation. The treatment worked across all stages of healing but proved most effective during early blastema formation, when stem-like cells are still deciding their fate. Chemical inhibition of RA synthesis blocked repair in rabbits, confirming that insufficient production of RA, prompted by the deficiency of rate-limiting enzyme Aldh1a2 and enhanced degradation, was responsible for the failure of ear pinna regeneration in rodents.
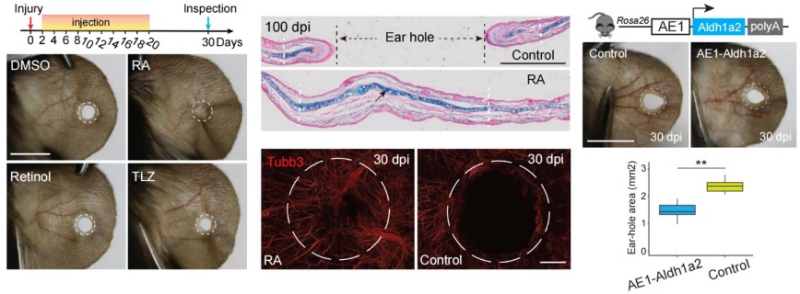 Retinoic acid injections and transgenic rabbit enhancer insertion both restore ear regeneration in mice with complete cartilage and nerve regrowth. These breakthroughs prove dormant regenerative programs can be reactivated for human therapeutic applications.
Retinoic acid injections and transgenic rabbit enhancer insertion both restore ear regeneration in mice with complete cartilage and nerve regrowth. These breakthroughs prove dormant regenerative programs can be reactivated for human therapeutic applications.
The therapeutic implications stretch far beyond mouse ears. Retinoic acid is already FDA-approved for treating certain cancers and skin conditions, meaning that clinical trials for regenerative applications could potentially fast-track through regulatory approval. The research team showed that the same molecular pathway is active during regeneration in fish fins and salamander limbs, suggesting that RA-based therapies might work across diverse tissue types. For trauma surgeons treating battlefield injuries, plastic surgeons reconstructing birth defects, or cardiologists facing heart attack patients, the prospect of flipping a single molecular switch to restore rather than merely repair represents a paradigm shift in medicine.
Beyond solving a long-standing biological puzzle, the work places the Aldh1a2/retinoic-acid axis on the therapeutic radar for trauma repair, orthopaedic surgery and perhaps digit regeneration. The research team invites trauma surgeons, regenerative medicine researchers, and pharmaceutical companies to explore how the retinoic acid pathway might translate to human applications. All raw spatial and single-cell data have been deposited at public data repository for community exploration.
This research can be accessed here: https://www.science.org/doi/10.1126/science.adp0176



