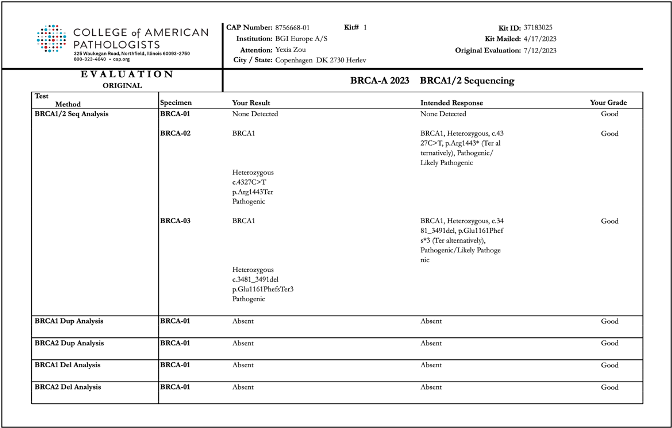
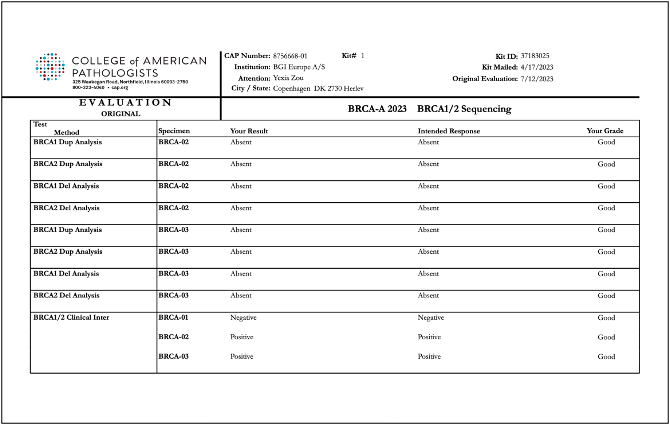
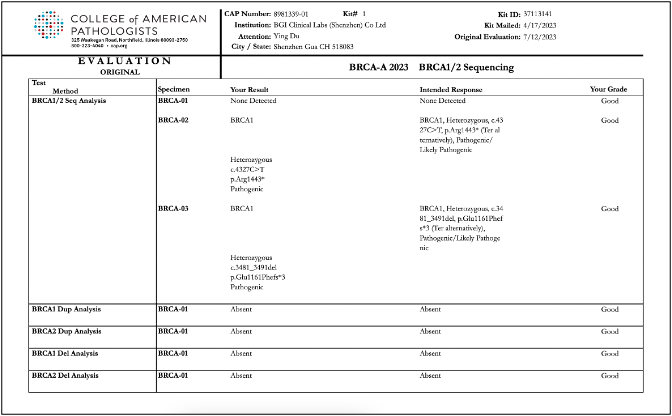
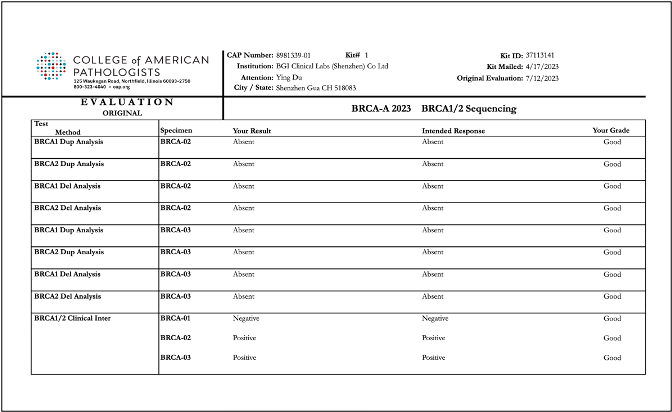
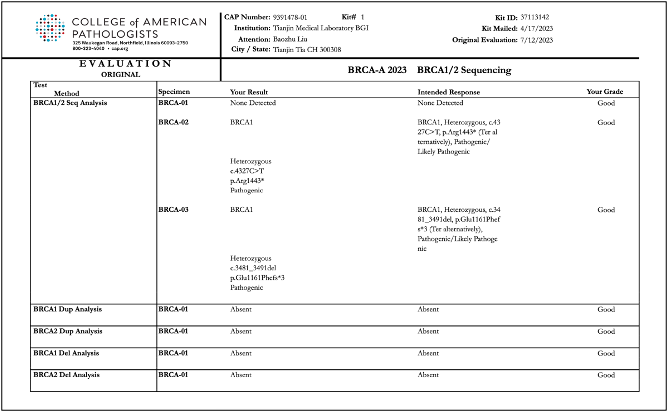
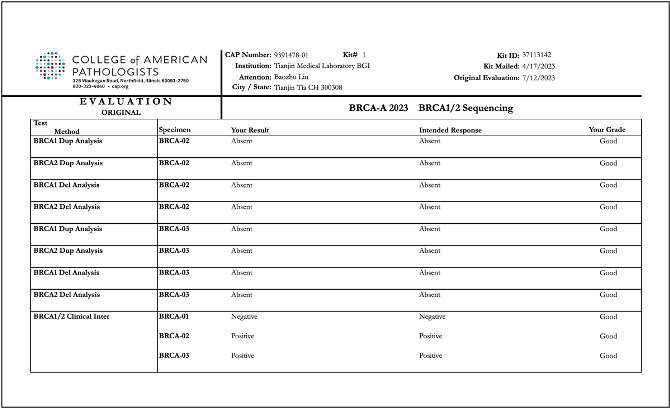
Recently, BGI Genomics, a subsidiary of BGI Group, achieved "Good" ratings in all assessed categories in the College of American Pathologists (CAP) BRCA-A 2023 BRCA1/2 Sequencing evaluation program at three of its medical testing laboratories located in Denmark, Shenzhen, and Tianjin. This accomplishment serves as strong evidence of the company’s ability to maintaining international standards in testing procedures and ensuring the accuracy of BRCA gene germline and somatic testing.
To conduct the evaluation, BGI utilized its proprietary BRCA1/2 gene testing reagent kits applied on high-throughput sequencing platforms. The program yielded consistent and reliable results across all laboratories, further validating the reliability of BGI's technology in conducting clinical testing for oncology genes.
The BRCA1 (BReast CAncer gene 1) and BRCA2 (BReast CAncer gene 2) genes are the most common affected genes in hereditary breast and ovarian cancer. Inherited mutations in these genes are responsible for approximately 3% of breast cancers (affecting about 7,500 women per year) and 10% of ovarian cancers (affecting about 2,000 women per year). As awareness of genetic testing growing among the public, BRCA1/2 gene testing has garnered increasing attention in clinical practice. However, due to its non-hotspot mutation nature, which poses significant challenges to the entire test, including experimental procedures, analysis and interpretation.
CAP BRCA1/2 Sequencing is a comprehensive interlaboratory proficiency evaluation program designed to assess the detection capability of global genetic testing laboratories in identifying BRCA gene variations. The program comprehensively evaluates laboratories' proficiency in detecting various types of mutations, such as point mutations, insertions, deletions, and large genomic rearrangements in BRCA1/2 genes, as well as their capacity to predict nucleic acid and protein variations. Additionally, it also assesses laboratories' compliance and accuracy in conducting bioinformatics analysis and report interpretation. These assessments are carried out twice a year.
Established in 1947, the College of American Pathologists (CAP) stands as the foremost organization of board-certified pathologists, committed to promoting and championing excellence in the fields of pathology and laboratory medicine on a global scale. CAP's Accreditation Programs are founded on a unique, reciprocal, peer-based inspection model that brings benefits to both the laboratories being inspected and the laboratories providing the inspection.
The outcome of this evaluation reinforces BGI's commitment to upholding the highest standards in genetic testing, further solidifying its prominent position in the global genomics sector. As the demand for advanced genetic testing solutions continues growing, BGI remains dedicated to advancing the field of genomics and providing reliable and accurate testing services for the benefit of patients and healthcare professionals worldwide.
Reports of CAP BRCA-A 2023 BRCA1/2 Sequencing evaluation program for BGI Genomics medical testing laboratories in Denmark, Shenzhen, and Tianjin: