Co-led by BGI-Research, a recent study published by Cancer Discovery reveals the interactions between tumor cells and the immune microenvironment in intrahepatic cholangiocarcinoma (iCCA). The research deepens the current understanding of tumor development, drug resistance and immune tolerance, providing novel evidence for individualized treatment strategies of targeted therapy and immunotherapy.
 Geospatial immune heterogeneity reflects the diverse tumor-immune interactions in intrahepatic cholangiocarcinoma published in Cancer Discovery.
Geospatial immune heterogeneity reflects the diverse tumor-immune interactions in intrahepatic cholangiocarcinoma published in Cancer Discovery.
iCCA is the second most common primary liver cancer with increasing incidence worldwide. Characterized by high invasiveness and frequent postoperative recurrence, iCCA exhibits one of the highest mortality rates among human cancers. Surgery is the only radical treatment for early-stage patients, however, nearly 70% of iCCA patients experience tumor recurrence and metastasis after surgery, resulting in the low five-year survival rate with 20% to 40% after surgery. Immunotherapy has revolutionized the standard treatment for cancer patients in recent years. However, the overall treatment response is poor because of the immune escape and drug resistance in tumor.
Tumors contain not only a large number of malignant tumor cells, but also immune cells, stromal cells, micro vessels, etc. They constitute a comprehensive ecosystem together, called tumor microenvironment. Infiltrated immune cells hold the potential of killing malignant cells, representing the major external selection pressure to sculpt the tumor evolution. In response, tumor cells can escape from the elimination in various ways, and even strike back by inducing the exhaustion of immune cells. Different tumors have distinct immune microenvironment characteristics and also immune escape mechanisms, leading to different responses to immunotherapy.
In this study, the researchers performed whole exome and transcriptome sequencing of 207 iCCA samples from 45 patients and found that different regions displayed extremely high heterogeneity at both genomic and immune microenvironment levels. Notably, over 40% of the gene mutations and copy number alterations were distinct among tumor subregions within the same patients. Meanwhile, more than half of iCCA displayed intratumoral heterogeneous immune infiltration, posing a great challenge for the immune classification of patients and the prediction of immunotherapeutic efficacy by single biopsy.
Based on the immune infiltration across tumor subregions, researchers divided iCCA patients into sparsely infiltrated, heterogeneously infiltrated, and highly infiltrated immune groups. Distinct immunogenomic features and immune escape mechanisms were characterized, hinting the different treatment strategies for three immune groups.
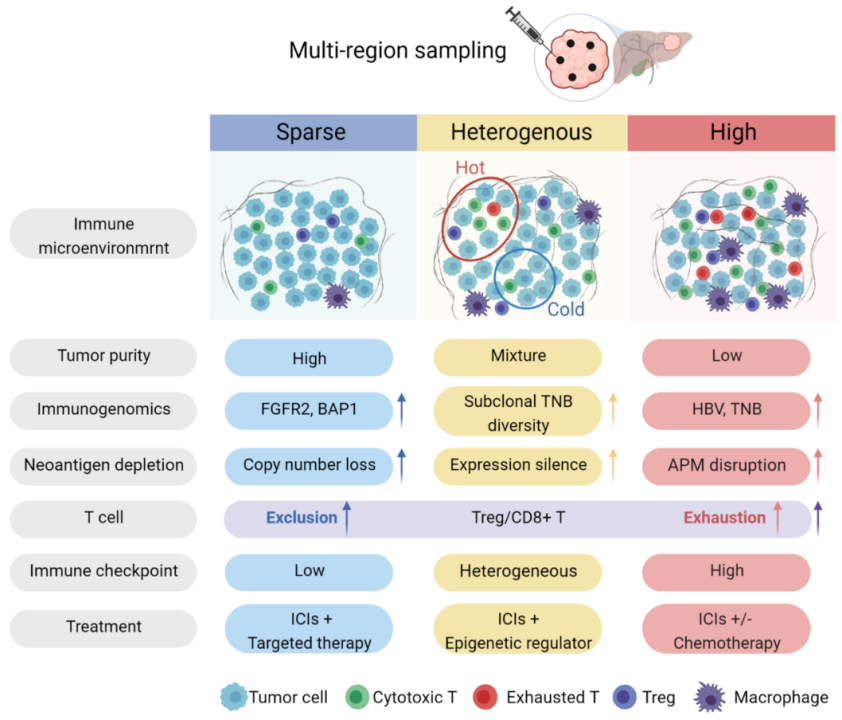 A classification system developed by the study to divide iCCA patients into highly, sparsely, and heterogeneously infiltrated immune groups.
A classification system developed by the study to divide iCCA patients into highly, sparsely, and heterogeneously infiltrated immune groups.
Highly infiltrated tumor: “cunning” tumor cells vs. exhausted immune cells
Although abundant immune cells have infiltrated into the tumor, most are in a state of exhaustion and cannot perform their function to kill the tumor cells. At the same time, tumor cells have a higher frequency of loss of heterozygosity of HLA (human leukocyte antigen) and copy number loss of antigen-presentation genes to escape from the recognition by the immune system. A combination of immunotherapy and chemotherapy is suggested for this kind of tumor.
Sparsely infiltrated tumor: “camouflaged” tumor cells vs. insufficient immune cells
This type of tumor contains fewer infiltrated immune cells but a large number of malignant tumor cells with specific driver mutations. Tumor cells show active copy number loss of clonal neoantigens to reduce immunogenicity. Such patients may need targeted therapy combined with immunotherapy.
Heterogeneously infiltrated tumor: both of the above
Heterogeneously infiltrated tumor has both subregions with high immune infiltration (Hot) and sparse immune infiltration (Cold). Due to the reduced expression of clonal neoantigens, immunotherapy combined with epigenetic regulators is suggested for such patients.
“This work integrates multiple research methods to delineate the dynamic interactions between tumor cells and immune microenvironment in iCCA, revealing the impact of driver mutations on the immune microenvironment and the multiple neoantigen depletion mechanisms, and providing insights for the understanding of tumor immune evasion and the development of novel immunotherapy strategies.” said Dr. Kui Wu, joint corresponding author of the study and Researcher at BGI-Research.
Researchers from Zhongshan Hospital of Fudan University and China Academy of Science also participated in this study. Ethical approval was received for the research.



