In a remarkable scientific venture, researchers from BGI-Research, Peking Union Medical College Hospital, and Shenzhen Luohu District People's Hospital collaborated to explore the world of microbes within surgically created neovagina. Their groundbreaking study, published in Nature Communications, focused on women diagnosed with Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome, a congenital condition characterized by the underdevelopment or absence of the vagina. The study utilized the neovagina in MRKH patients as a model to investigate how a microbial community develops in such an environment from a nearly blank ecological niche.
 The research “Insights into the assembly of the neovaginal microbiota in Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome patients” was published in Nature Communications.
The research “Insights into the assembly of the neovaginal microbiota in Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome patients” was published in Nature Communications.
Many existing studies have shown that the vaginal microbiota is crucial to women's reproductive health. However, until now, it has been difficult to find an appropriate model to explore the mechanisms by which the microbiota is established in this area.
The research team followed 39 women with MRKH syndrome who had undergone laparoscopic peritoneal vaginoplasty - a surgical procedure to create a vagina - over a period of up to four years. This involved multiple sampling points and detailed metagenomic sequencing to analyse the microbial composition at various stages before, during, and after the surgery. This longitudinal approach allowed the team to map out the evolution of the microbial community within the newly formed vaginal environment.
Navigating through the study was not without its challenges. The research coincided with the global COVID-19 pandemic, which added layers of complexity to the follow-up process. Moreover, the sensitivity of the subject and the personal transitions of the patients - such as entering relationships or getting married—made long-term follow-ups particularly difficult. Despite these obstacles, about 70% of the patients participated in the final phase of the study, providing critical data that enriched the research findings.
The study revealed that the initial microbial community in the neovagina was almost a clean slate, with adjacent areas like the original dimple (the pre-surgery external genital and the opening area of the neovagina) and the anus area serving as the primary “species pool”. Over time, this environment underwent significant changes. Squamous epithelial cells proliferated and spread, gradually covering the internal surfaces and mimicking the structure of a natural vagina. This process also shifted the nutritional landscape, making it conducive for different microbial species to flourish.
The researchers found that the artificial vagina's microbial community transits from a barren state to one resembling the microbial makeup of reproductive-age women, complete with increasing amounts of Lactobacillus – a bacterial genus associated with vaginal health. This transition was influenced by the initial “seeding” of microbes and the selective pressures of the new environment, showcasing a dynamic interplay that mirrors natural vaginal development.
At 14 days post-surgery, the microbiota of the neovagina exhibited more random characteristics, with significant individual differences and noticeable proliferation of some antibiotic-resistant bacteria. By 3 months post-surgery, the microbial community gradually began to resemble the structure of a normal vaginal microbiota, though it was more similar to the state of healthy postmenopausal women. As nutritional conditions progressively improved, the neovagina began to approach the status of healthy women of reproductive age, with Lactobacillus levels gradually increasing. Within 2 to 4 years post-surgery, the microbiota community evolved into a stable structure similar to the preoperative dimple microbiota.
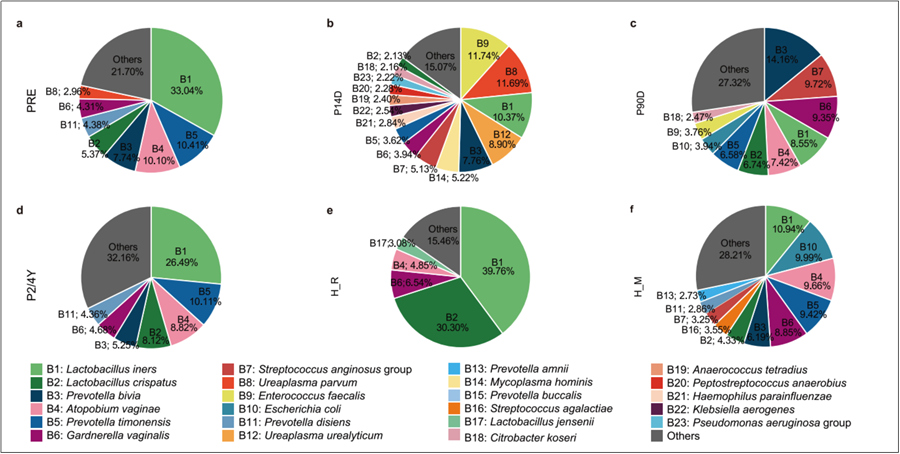 Trends in Vaginal Microbiota Changes Over Multiple Time Periods Before and After Surgery in Women with MRKH, Compared to Healthy Women.
Trends in Vaginal Microbiota Changes Over Multiple Time Periods Before and After Surgery in Women with MRKH, Compared to Healthy Women.
A particularly significant finding was the role of Lactobacillus crispatus, which primarily originated from the pre-surgery dimple. Introducing this bacterium before or early after the surgery could potentially improve the microbial structure and enhance the overall health of the neovagina. This insight provides a promising avenue for therapeutic interventions aimed at establishing a healthy microbial community in neovaginas, offering a better postoperative prognosis for patients with MRKH syndrome and transgender women undergoing similar surgeries.
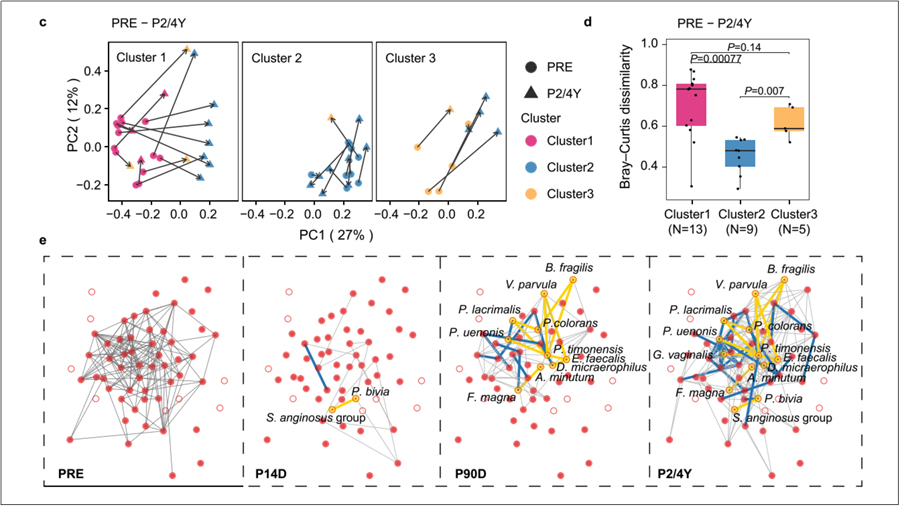 Ecological Evolution of the Artificial Vaginal Microbial Community in Women with MRKH.
Ecological Evolution of the Artificial Vaginal Microbial Community in Women with MRKH.
"This study found that the colonization of the most important probiotic in healthy women – L. crispatus - in the neovagina primarily originates from the pre-surgery dimple. This suggests that introducing the L. crispatus strains into the dimple before surgery or into the neovagina in the early postoperative period may help promote the colonization of this beneficial bacterium and facilitate the formation of the postoperative microbiota,” explained Dr. Chen Chen, a researcher at BGI-Research and a corresponding author of the paper.
“This provides crucial scientific evidence for improving the vaginal microbiota in women with MRKH syndrome and transgender women and offers significant guidance for postoperative management.”
The research team extends its deepest gratitude to Medical Professor Luo Guangnan, founder of the " Luohu’s vaginoplasty procedure," and his team for their inspirational dedication. Even in his 80s, Professor Luo personally conducted patient follow-ups, demonstrating exceptional commitment. His passing before the publication of this paper is a deeply regrettable loss. This study is intended to honor his legacy of passion for medical research and patient care.
Ethical review approval was obtained for this study.
For more details, please go to the paper https://www.nature.com/articles/s41467-024-52102-1 or the story behind the paper https://communities.springernature.com/posts/a-collaborative-journey-in-unraveling-the-assembly-of-neovaginal-microbiota-in-mrkh-patients-0b4a7cf7-f193-44dc-8e93-d8ffa91ee479



