BGI-Research, in cooperation with Lamprey Research Center of Liaoning Normal University and other institutions, utilized BGI’s single-cell RNA sequencing (scRNA-seq) and single-nucleus RNA sequencing (snRNA-seq) technologies and sequencing platform to construct the most completed single-cell transcriptome atlas of lamprey (Lethenteron reissneri). This research systematically analyzed the cellular diversity and its evolutionary features of the lamprey’s immune system and lipid metabolism. Researchers have also discovered a unique protein, NATTERIN, in lamprey, which has shown potential for promoting white adipose tissue (WAT) browning. This research was published in Nature Communications.

The study “Single-cell transcriptome atlas of lamprey exploring Natterin- induced white adipose tissue browning” was published in Nature Communications.
Lampreys are primitive, jawless vertebrates that predominantly inhabit coastal and fresh water environments, thriving across most temperate regions worldwide. Their unique evolutionary position, bridging invertebrates and jawed vertebrates, makes them an ideal model for studying vertebrate evolution. However, the lack of cytomic research on various lamprey organs has impeded advancements in this field.
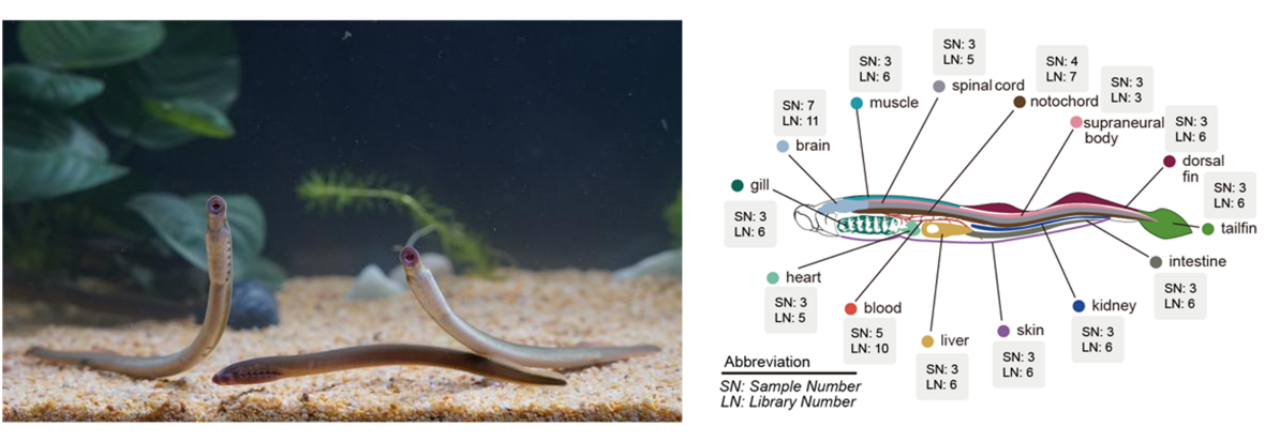 Anatomy of the lamprey and single-cell sampling of its tissues.
Anatomy of the lamprey and single-cell sampling of its tissues.
In this study, researchers successfully generated the most complete cellular atlas of the lamprey to date. By performing single-cell sequencing on samples from 14 tissues including blood, muscle, liver, heart, intestine from 30 lamprey larvae, the research team analyzed a total of 604,460 cells/nuclei and identified 70 distinct cell types. Among these, 22 cell types (e.g., endothelial cells and various immune cells) were found across different tissues, and 48 cell types were identified as tissue-specific.
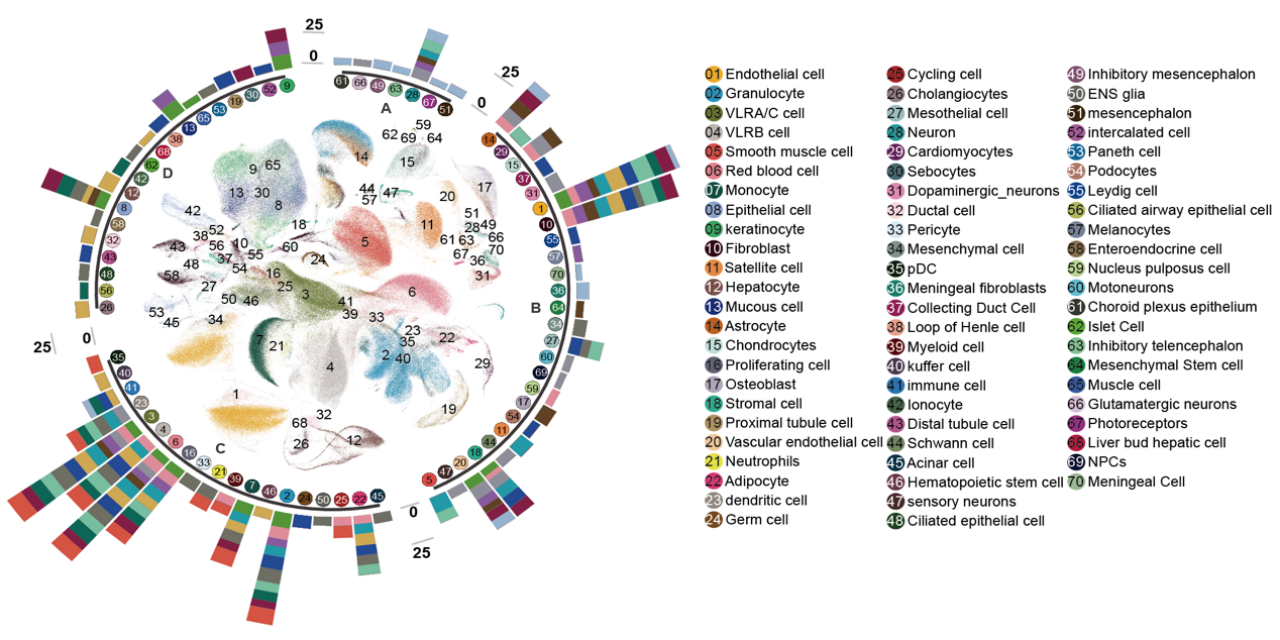 UMAP visualization of cell types across 14 lamprey tissues (inner) with a bar plot showing cell type counts (outer).
UMAP visualization of cell types across 14 lamprey tissues (inner) with a bar plot showing cell type counts (outer).
The research illustrated the presence of islet-like and acinar-like cells in the gut of the lamprey. The researchers found significant similarities in their cellular features by comparing the cell types of lampreys with those of mice and humans. Although lampreys do not have a typical pancreas, the study identified 92 islet-like cells (expressing the marker genes Ins, Cpe, and Epas1) and 689 acinar-like cells (expressing the marker genes Ctrb1 and Cel) in the intestine. These cells resemble pancreatic cells in mice and humans, suggesting that the lamprey intestine may perform some functions similar to those of the pancreas.
The research team conducted an in-depth analysis of the immune cells of the lamprey and identified a total of 280,636 immune cells. Among these, the team discovered a key immune-related protein, NATTERIN, which is highly expressed in the lamprey’s granulocytes and localized in lipid droplets. Further studies revealed that NATTERIN is not only a component of the innate immune system but may also be involved in regulating lipid metabolism, especially playing a key role in the browning process of white fat.
To investigate the function of NATTERIN, the research team created a Natterin transgenic model in mice. An obesity model was then created by inducing a high-fat diet to assess the role of NATTERIN in adipose tissue. In the inguinal WAT of the mice overexpressing NATTERIN, the research team observed numerous smaller multilocular lipid droplets resembling those in brown adipocytes. This conversion of WAT into brown adipose tissue (BAT) was further confirmed by the high expression level of UCP1, a protein that facilitates mitochondrial thermogenesis by uncoupling oxidative phosphorylation, allowing energy to be dissipated as heat rather than stored as ATP. This thermogenic process plays a crucial role in energy metabolism and heat production, potentially increasing metabolic rate and energy expenditure. While BAT activation has been associated with improved metabolic health, its direct role in weight loss and obesity prevention requires further investigation.
The team also administered low dose NATTERIN injections (subcutaneous, 125 µg/kg) and found that it induced weight loss effect similar to Orlistat. Moreover, the low dose of NATTERIN showed no significant toxicity and did not cause inflammation or organ damage, suggesting its safety and potential therapeutic value in metabolic regulation.
Dr. Guangyi Fan, the corresponding author of the study and a researcher at BGI-Research, stated, "In this study, we have constructed the most comprehensive cell atlas of lamprey organs, which provides unprecedented data to support our understanding of vertebrate evolution. Additionally, the role of NATTERIN proteins in lipid metabolism opens up new therapeutic targets for metabolic diseases."
The findings of this research are expected to offer new insights across various fields, such as vertebrate evolution, immune system function analysis, fat metabolism regulation, and the development of new obesity intervention strategies.
Ethical review approval was obtained for this study.
This study can be accessed here: https://www.nature.com/articles/s41467-025-56153-w



