July 25, Shenzhen – Clinical research published in Nature Medicine and co-led by BGI-Research has demonstrated the safety and feasibility of a neoadjuvant immunotherapy medication for patients with locally advanced esophageal squamous cell carcinoma (ESCC), as well as introduced a crucial treatment response indicator, identified three tumor microenvironment phenotypes relevant to immunotherapy, and examined the reasons behind treatment efficacy or non-efficacy in specific patients.
 The research paper “Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial” is published in Nature Medicine.
The research paper “Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial” is published in Nature Medicine.
Dr. Wu Kui, co-correspondent author and researcher at BGI-Research, commented that this research offers valuable guidance for conducting larger-scale clinical trials, improving the precision diagnosis and treatment of ESCC, and optimizing efficacy to enhance patient survival.
ESCC is a malignant tumor that affects the digestive system and is one of the most prevalent forms of esophageal cancer worldwide. The 5-year survival rate declines dramatically based on clinical stage, ranging from 52.7% in stage 0 to only 3.4% in the advanced stage. Unfortunately, most patients with esophageal cancer receive their diagnosis at an advanced stage, primarily due to the nonspecific nature of early symptoms.
The current clinical standard treatment involves neoadjuvant chemoradiotherapy or chemotherapy followed by surgery. Neoadjuvant treatment is therapy to shrink the tumor before the main treatment, typically surgery, is administered.
In this study, the new neoadjuvant treatment using an anti-PD-L1 medication called Adebrelimab demonstrated promising results. It caused no severe side effects, and 24% of the patients in the study group experienced significant tumor shrinkage. The two-year overall survival rates were 92%, and 100% of patients showed no disease recurrence in this period. This treatment proved to be significantly superior to chemotherapy or chemoradiotherapy in both safety and effectiveness.
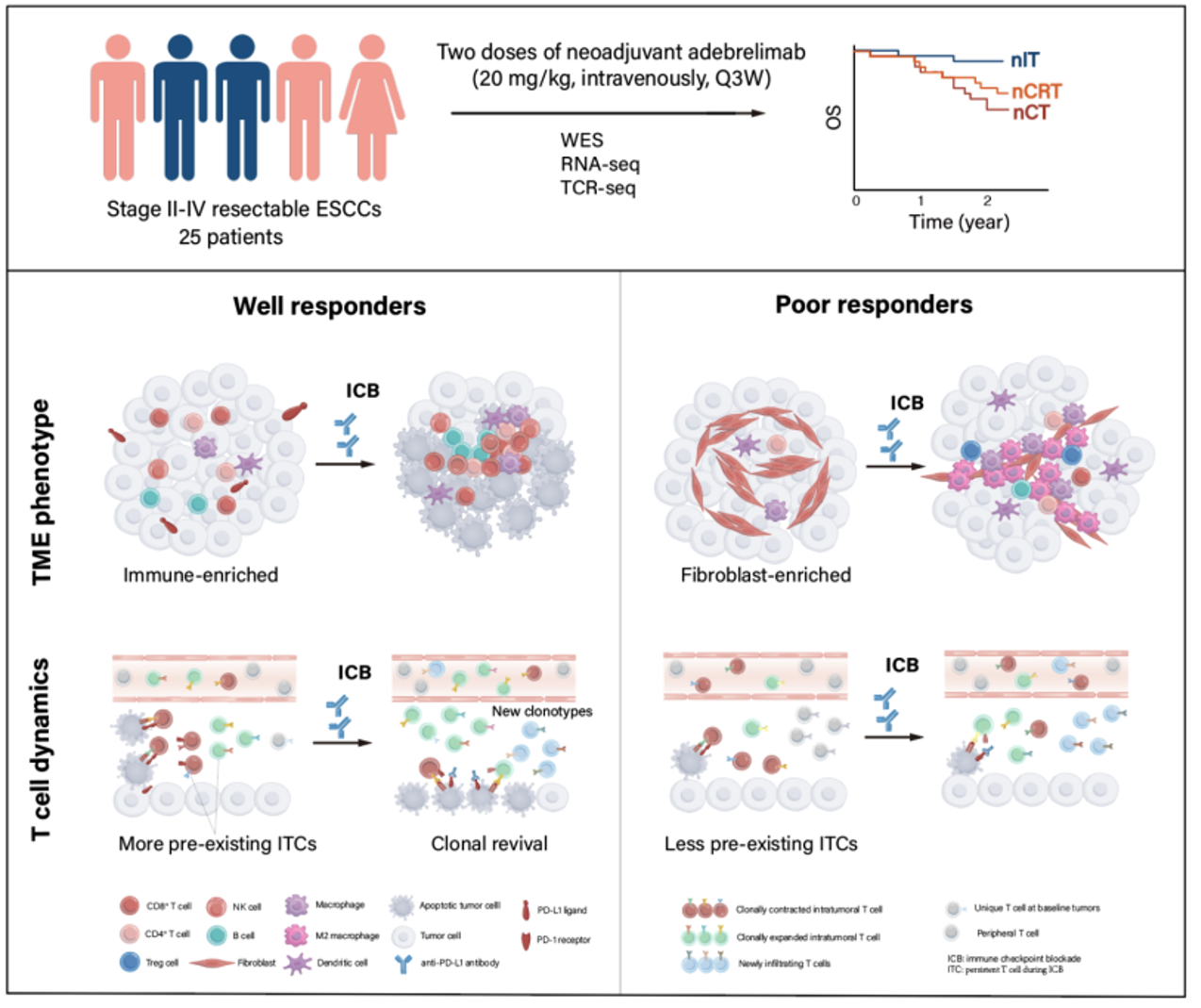 Graphical abstract of the study
Graphical abstract of the study
The research team compared patients who responded well to the treatment (well responders) with those who responded poorly (poor responders). They analyzed potential biomarkers related to treatment effectiveness. Although no significant differences in genetic mutations related to treatment response were found, the researchers utilized various types of data to create an indicator based on 12-gene signature from transcriptome data. This score effectively predicts the response to immunotherapy for patients.
Furthermore, the researchers identified three immune microenvironment phenotypes: Immune-enriched, Tumor-proliferation, and Fibroblast-enriched. Patients with an "Immune-enriched" phenotype demonstrated more significant tumor shrinkage compared to the other two types, and a higher proportion of patients in the well responder group exhibited the "Immune-enriched" phenotype.
The analysis of the poor responders group revealed that the patients' tumors had a higher concentration of immune-suppressive cells, which remained unable to transform into the "Immune-enriched" phenotype even after undergoing immunotherapy. The research team further studied two patients who responded poorly and found that, although immunotherapy activated immune cells (T cells), it couldn't fully trigger the cancer immunity cycle, indicating the necessity of developing new treatment strategies for these patients.
Through comparative analysis, researchers discovered that patients who underwent treatment in the well responder group exhibited a significant increase in the diversity of T cell receptors (TCR) within their tumors after undergoing immunotherapy, which was positively correlated with tumor shrinkage. The researchers also identified a group of pre-existing intratumoral T cells, known as ITCs, which underwent significant clonal expansion following immunotherapy. Alongside other newly infiltrating T cells inside the tumor, they formed a group of tumor-reactive T cells that demonstrated anti-tumor immune killing effects.
Additionally, the researchers found that the immune response successfully eliminated most tumor antigens recognized by high-affinity ITCs in the well responding patients. This further proves that the ITCs in the well responder group possess superior abilities to recognize tumor neoantigens and activate the immune response.
This study marks the world's first phase 1b clinical trial of neoadjuvant mono-immunotherapy for locally advanced esophageal squamous cell carcinoma, and provides compelling evidence to support the use of anti-PD-L1 monotherapy as a groundbreaking treatment approach for esophageal squamous cell carcinoma.
The project underwent ethical review and adheres to regulations, and privacy protocols.
Read the article:
https://www.nature.com/articles/s41591-023-02469-3



