Co-led by BGI-Research and Sun Yat-sen University, a recent study has made a significant discovery in the field of cancer immunotherapy. Using BGI’s cutting-edge high-resolution spatial transcriptomics technology, Stereo-seq, scientists have, for the first time, mapped the panoramic development of tertiary lymphoid structures (TLSs) in hepatocellular carcinoma (HCC), the most common type of primary liver cancer. The study not only provides a detailed characterization of the unique features of TLSs in orchestrating antitumor immunity and modulating responses to immunotherapy but also reveals, for the first time, the critical regulatory role of tryptophan metabolism in TLS maturation. The study was published in Cancer Cell.
 The study, titled “Spatial transcriptomics reveals tryptophan metabolism restricting maturation of intratumoral tertiary lymphoid structures,” was published in Cancer Cell.
The study, titled “Spatial transcriptomics reveals tryptophan metabolism restricting maturation of intratumoral tertiary lymphoid structures,” was published in Cancer Cell.
The research focused on tertiary lymphoid structures (TLSs) - ectopic lymphoid aggregates that form within tumors in response to immunological stimuli and orchestrate antitumor immune responses. Unlike lymph nodes, TLSs appear directly within inflamed or cancerous tissues and act as localized immune "command centers" to help the body fight cancer. Previous studies have shown that patients with well-developed TLSs respond better to immune-modulating therapies, such as anti-PD-1 therapy. However, these structures remain immature in many patients, failing to provide strong immune support. Why TLSs often fail to mature inside tumors and how to encourage their development remained largely unknown, prompting the team to pursue this research.
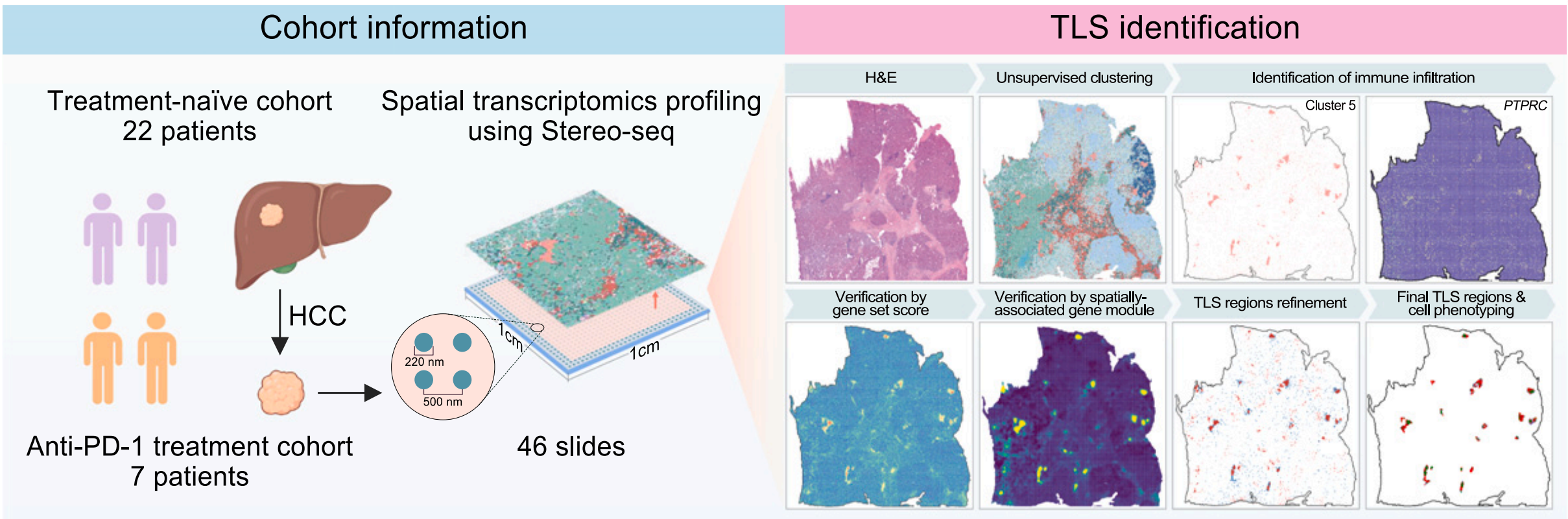 The TLS identification workflow designed for this study successfully enabled in situ identification of TLSs in HCC tissue.
The TLS identification workflow designed for this study successfully enabled in situ identification of TLSs in HCC tissue.
Using Stereo-seq, the research team successfully identified a total of 937 TLSs, including 69 mature TLSs. Previous studies have demonstrated that mature TLSs are positively associated with improved survival and enhanced responses to immunotherapy in various cancer types. However, the functional role of immature TLSs has remained unclear. To address this, the team developed a novel spatial developmental trajectory-based classification model that categorizes immature TLSs into two distinct types: conforming TLSs, which show potential to mature and support immune responses, and deviating TLSs, which appear developmentally stagnant.
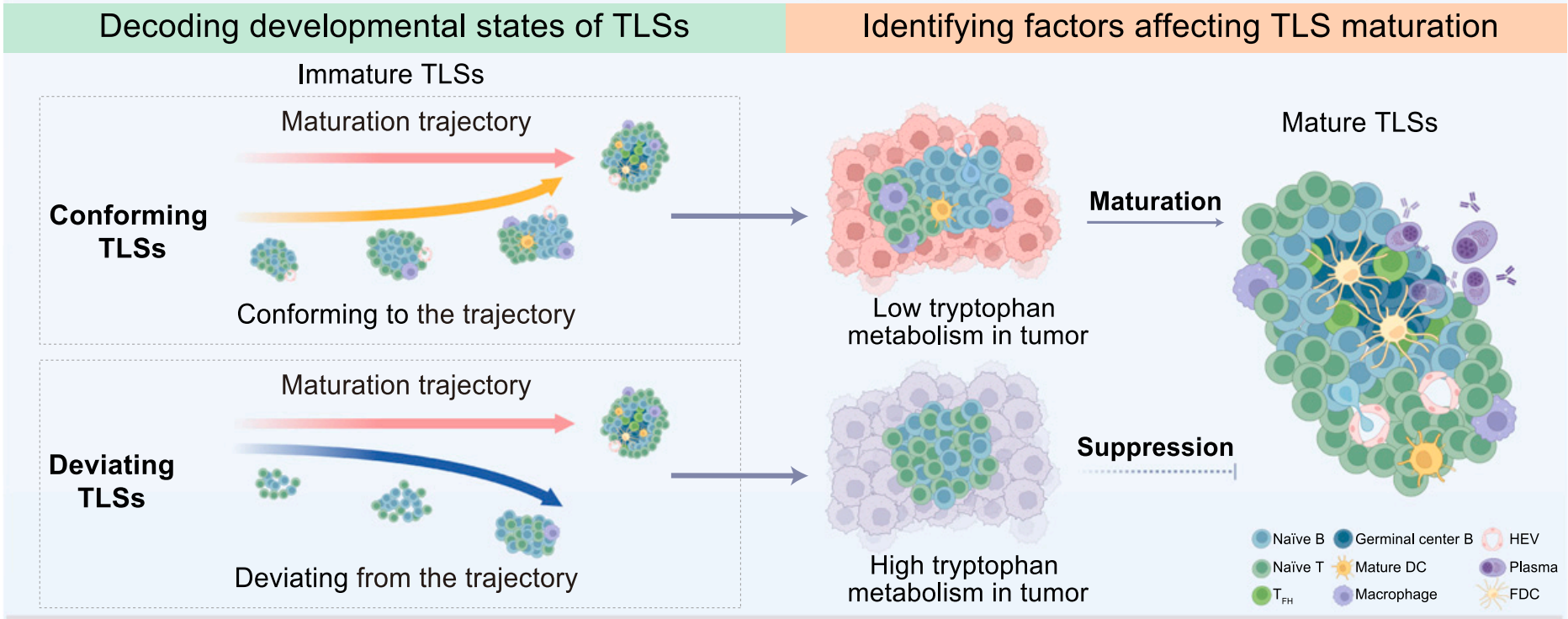 The different developmental pathways of immature TLSs are influenced by the levels of tryptophan metabolism in the tumor microenvironment.
The different developmental pathways of immature TLSs are influenced by the levels of tryptophan metabolism in the tumor microenvironment.
Researchers delved deeper into the reasons why TLSs deviate from their normal development and identified an unexpected factor: tryptophan metabolism. In TLSs that failed to mature properly, immune cells - especially B cells - exhibited unusually high activity in the tryptophan metabolic pathway, particularly in the presence of an enzyme called TDO2. Further investigation revealed that this heightened metabolism was not an internal property of the immune cells but was instead induced by surrounding cancer cells in the tumor microenvironment. Malignant cells produced large amounts of tryptophan-metabolizing enzymes, altering the local biochemical environment and effectively suppressing the immune system's ability to build mature TLSs.
In a mouse model, the team further validated this finding: a low-tryptophan diet significantly increased the density of mature TLSs and improved the metabolic environment within tumors. Cellular and tissue analysis results showed that both a low-tryptophan diet and TDO2 inhibitors increased the number of mature TLSs and synergized with anti-PD-1 treatment to effectively reduce tumor volume. These findings emphasize the critical role of tryptophan metabolism in determining the maturation of TLSs and their response to immunotherapy.
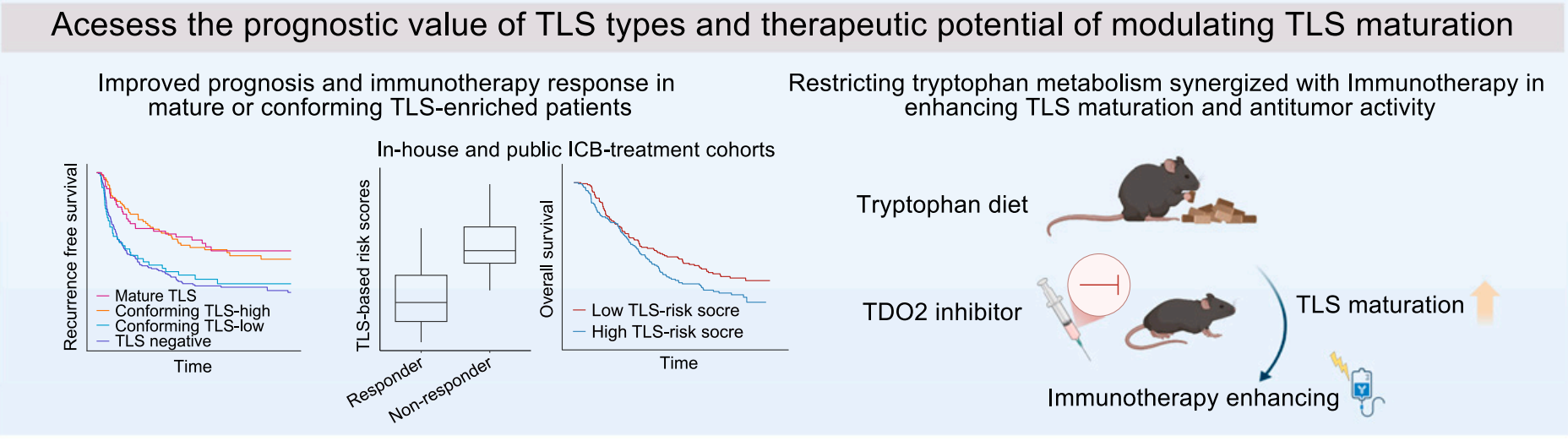 The study shows that conforming TLSs have the potential to respond to immunotherapy. Restricting tryptophan metabolism and reducing tryptophan intake can synergize with immunotherapy to enhance TLS maturation and antitumor activity.
The study shows that conforming TLSs have the potential to respond to immunotherapy. Restricting tryptophan metabolism and reducing tryptophan intake can synergize with immunotherapy to enhance TLS maturation and antitumor activity.
Dr. Yinqi Bai, the first author of the study and Chief Scientist at BGI-Research, stated: “This study not only elucidates the intrinsic link between TLS maturation and antitumor immunity but also highlights the critical role of metabolic pathways in the tumor microenvironment in regulating immune cell functions. It provides a new perspective on why some patients respond well to immunotherapy, while also pointing to industrial directions for the early diagnosis of HCC and other cancers, prediction of therapeutic responses, and the development of novel immunotherapy strategies. This research demonstrates broad prospects for clinical translation and application.”
Professor Ming Kuang, the corresponding author of the study, Dean of the Zhongshan School of Medicine, and Vice President of the First Affiliated Hospital at Sun Yat-sen University, said: “We are currently focused on researching TLSs in tumors. High-resolution spatial transcriptomics technologies have provided us with a new tool to explore the tumor immune microenvironment, enabling us to gain insights into the intrinsic biological mechanisms of complex and irregular TLSs. Our research has revealed the decisive impact of tryptophan metabolism on TLS maturation in the tumor microenvironment, which holds significant implications for tumor immunotherapy.”
Written informed consent was obtained from all patients, and the study was conducted in compliance with all institutional ethical regulations.
The study can be accessed here: https://www.cell.com/cancer-cell/abstract/S1535-6108(25)00112-6



