In a recent study, scientists from the Cancer Hospital of the Chinese Academy of Medical Sciences and BGI-Research reported the most comprehensive investigation to date into the geospatial structure of different molecular subtypes of human breast cancer. The researchers used BGI’s state-of-the-art single-cell transcriptome platform and high-resolution spatial transcriptomics technology, Stereo-seq, to achieve these findings. This research provides critical information that could help optimize immunotherapy for breast cancer patients. The study was published in Nature Communications.
 The study “Spatially resolved of breast cancer uncovers intercellular machinery of venular niche governing lymphocyte extravasation” was published in Nature Communications.
The study “Spatially resolved of breast cancer uncovers intercellular machinery of venular niche governing lymphocyte extravasation” was published in Nature Communications.
Breast cancer is a highly heterogeneous disease, and despite the success of immune checkpoint inhibitors (ICIs) in treating specific subtypes, the overall efficacy of these therapies has been limited by inadequate immune infiltration in the tumor microenvironment (TME). A deeper understanding of the TME and the mechanisms that govern immune cell infiltration is crucial to improving therapeutic outcomes.
Current studies often consider venules as a single entity, concentrating on the interactions between venular endothelial cells (vECs) and immune or tumor cells. However, the role of venular smooth muscle cells (vSMCs), which are the closest partners of vECs, has been largely ignored due to limitations in existing technologies. Therefore, understanding the interactions between vECs and vSMCs, and how they work together to promote immune cell infiltration into tumors, is key to understanding tumor immune responses.
To investigate these mechanisms, the research team collected thirty samples from 23 patients, including 23 primary tumor samples and 7 paired metastatic lymph node samples, covering the four major molecular subtypes of Luminal A, Luminal B, HER2, and TNBC. By combining Stereo-seq and single-cell RNA sequencing (scRNA-seq) technologies, the team successfully constructed a detailed spatial atlas of breast cancer cells, identifying 13 major cell types and precisely mapping their spatial distribution characteristics in primary tumors and metastatic lymph nodes.
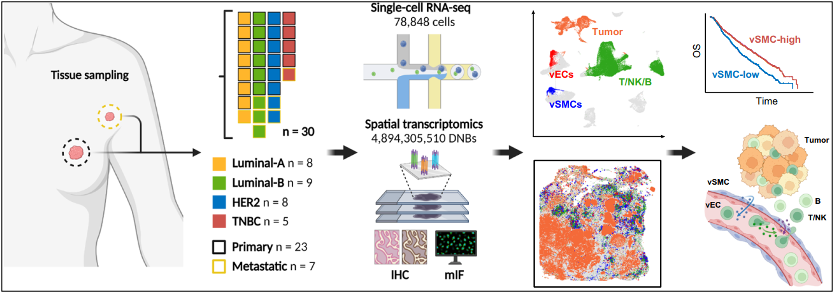 Schematic diagram illustrates the acquisition and analysis workflow of the spatial transcriptomics maps.
Schematic diagram illustrates the acquisition and analysis workflow of the spatial transcriptomics maps.
The research team found that endothelial cells (ECs) in the breast cancer TME exhibit significant functional and spatial heterogeneity, and can be categorized into three main subtypes: arterial ECs (aECs), capillary ECs (cECs), and venular ECs (vECs). Functional enrichment analysis revealed that, in addition to classic vascular functions, vECs specifically express higher levels of molecules involved in antigen presentation and immune activation signaling pathways, suggesting that vECs play an important immunoregulatory role in tumor immunity.
In addition, the study identifies vSMCs as the primary source of chemokines such as CCL21 and CCL19, which are responsible for recruiting lymphocytes. The chemokines secreted by vSMCs may subsequently be transported and presented through the atypical chemokine receptor ACKR1 on the surface of vECs. The close collaboration between vECs and vSMCs collectively forms a chemokine-rich "venular niche." These findings suggest that the venular niche may serve as a promising target to enhance the efficacy of breast cancer immunotherapy.
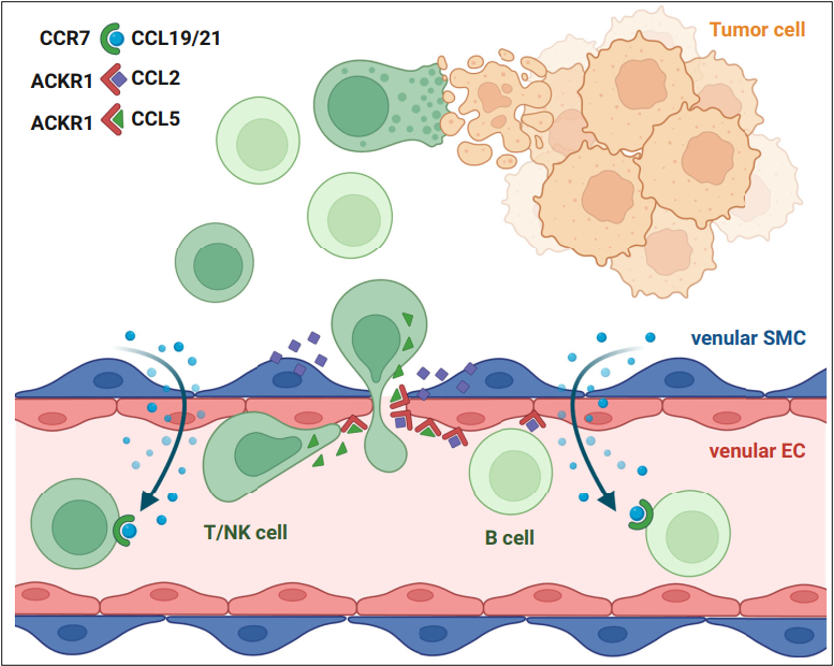 The hypothetical model of venular SMCs and venular ECs cooperating to increase lymphocyte extravasation.
The hypothetical model of venular SMCs and venular ECs cooperating to increase lymphocyte extravasation.
To explore the clinical significance of this finding, the research team analyzed the relationship between the expression levels of vECs and vSMCs signature genes and patient prognosis using the METABRIC and TCGA public databases, along with an independent validation cohort. The results consistently showed that a high proportion of vECs or vSMCs was significantly associated with better overall survival and lower recurrence risk in patients. Moreover, this correlation was independent of traditional prognostic factors such as age, tumor stage, and lymph node metastasis, suggesting that the abundance of the venular niche is an independent protective factor for breast cancer prognosis.
Ethical review approval was obtained for this study.
The study can be accessed here: https://www.nature.com/articles/s41467-025-58511-0



