On April 28, a team of scientists from BGI‑Research and Peking Union Medical College Hospital have drawn the first high‑resolution map of human carotid‑artery plaques. Harnessing the combined power of BGI's Stereo‑seq spatial transcriptomics and high‑throughput single‑cell RNA sequencing, they uncovered tiny immune "command posts" inside the plaque, structures the team calls plaque tertiary lymphoid‑organ‑like structures (PTLOs). Published in Nature Cardiovascular Research, the study shows that PTLOs drive a surge of plaque‑resident B‑cells that release potent IgG antibodies, turbo‑charge inflammatory macrophages and triple a patient's risk of stroke or transient ischaemic attack (TIA). By revealing where and how these immune hubs form, the work points to fresh ways of both predicting and preventing life‑threatening events.
 Published in Nature Cardiovascular Research, the study “Single-cell spatial transcriptomics of tertiary lymphoid organ-like structures in human atherosclerotic plaques” delivers the first spatially resolved single‑cell atlas of human carotid plaques and spotlights plaque tertiary lymphoid‑organ‑like structures (PTLOs) as hidden drivers of stroke‑prone inflammation.
Published in Nature Cardiovascular Research, the study “Single-cell spatial transcriptomics of tertiary lymphoid organ-like structures in human atherosclerotic plaques” delivers the first spatially resolved single‑cell atlas of human carotid plaques and spotlights plaque tertiary lymphoid‑organ‑like structures (PTLOs) as hidden drivers of stroke‑prone inflammation.
The team first profiled more than 100,000 individual plaque cells with the BGI’s single‑cell sequencing system, then layered on 10 ultra‑thin tissue sections analyzed by Stereo‑seq, BGI's chip‑based platform that preserves gene‑expression data at near‑histology resolution. Combining the two datasets produced a 14‑cell‑type atlas in which macrophages, smooth‑muscle cells and lymphocytes dominated. Crucially, the approach spotlighted compact lymphoid clusters that routine pathology had missed. Follow‑up immunofluorescence confirmed these as genuine lymphoid structures, while bulk RNA‑seq of laser‑micro‑dissected regions uncovered an antibody‑rich signature that sealed the PTLO identity. For patients, this means that high‑definition spatial omics is finally exposing the "enemy bases" that help plaques break open.
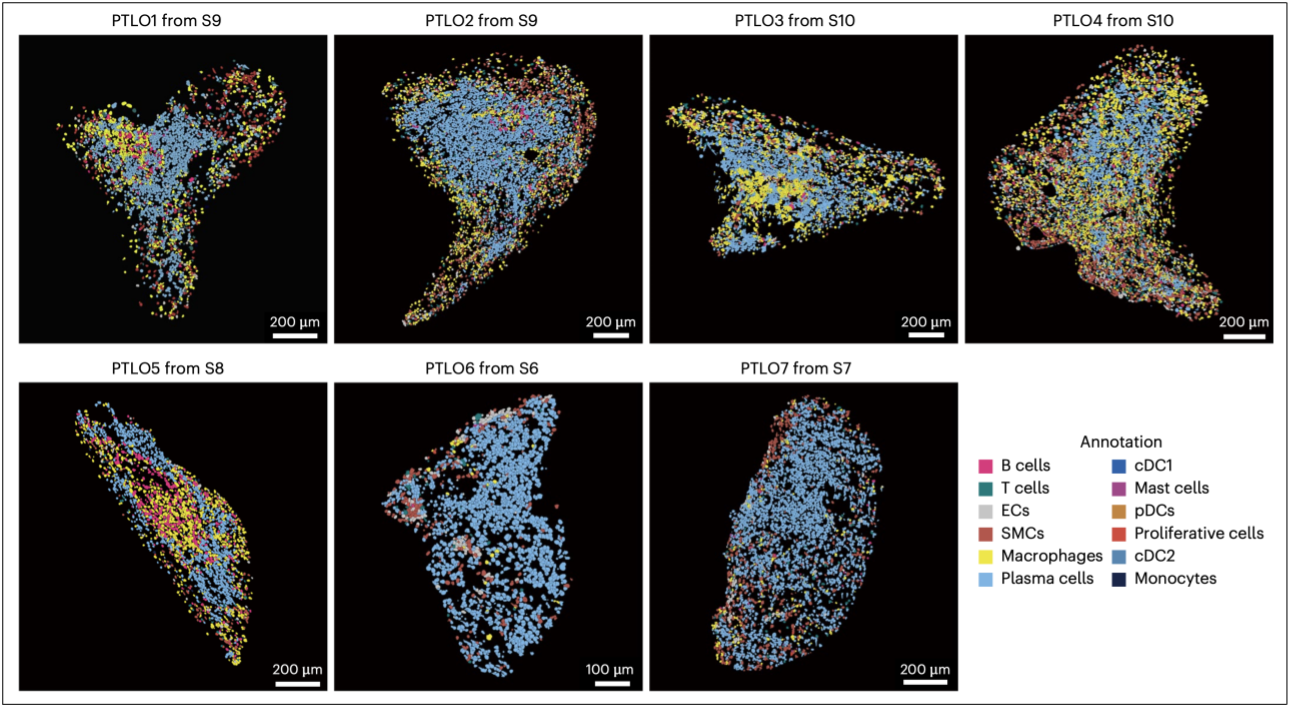 Stereo‑seq pinpoints seven plaque tertiary lymphoid‑organ‑like structures (PTLO1‑PTLO7); each dot is a single cell, colour‑coded by cell type.
Stereo‑seq pinpoints seven plaque tertiary lymphoid‑organ‑like structures (PTLO1‑PTLO7); each dot is a single cell, colour‑coded by cell type.
Digging deeper, the investigators found that a specialized group of fibroblast‑like smooth‑muscle cells (FLSMCs) sits at the heart of PTLO formation. By switching on the NF‑κB pathway, these cells pump out chemokines CXCL12 and CCL19 along with the "velcro" molecule VCAM‑1, signals that lure CXCR4‑positive B‑ and T‑cells into the niche. Once inside, B‑cells behave as if they were in a germinal centre: their receptors diversify, clones expand and plasma cells secrete class‑switched IgG, especially the IgG4 subtype. Stereo‑seq tracing shows those antibody‑secreting clones fanning out from PTLO cores into the surrounding plaque, where their antibodies latch onto FcγRIII (CD16) on macrophages and crank up inflammation. This chain of events clarifies how a single immune hotspot can set the stage for a major cardiovascular crisis.
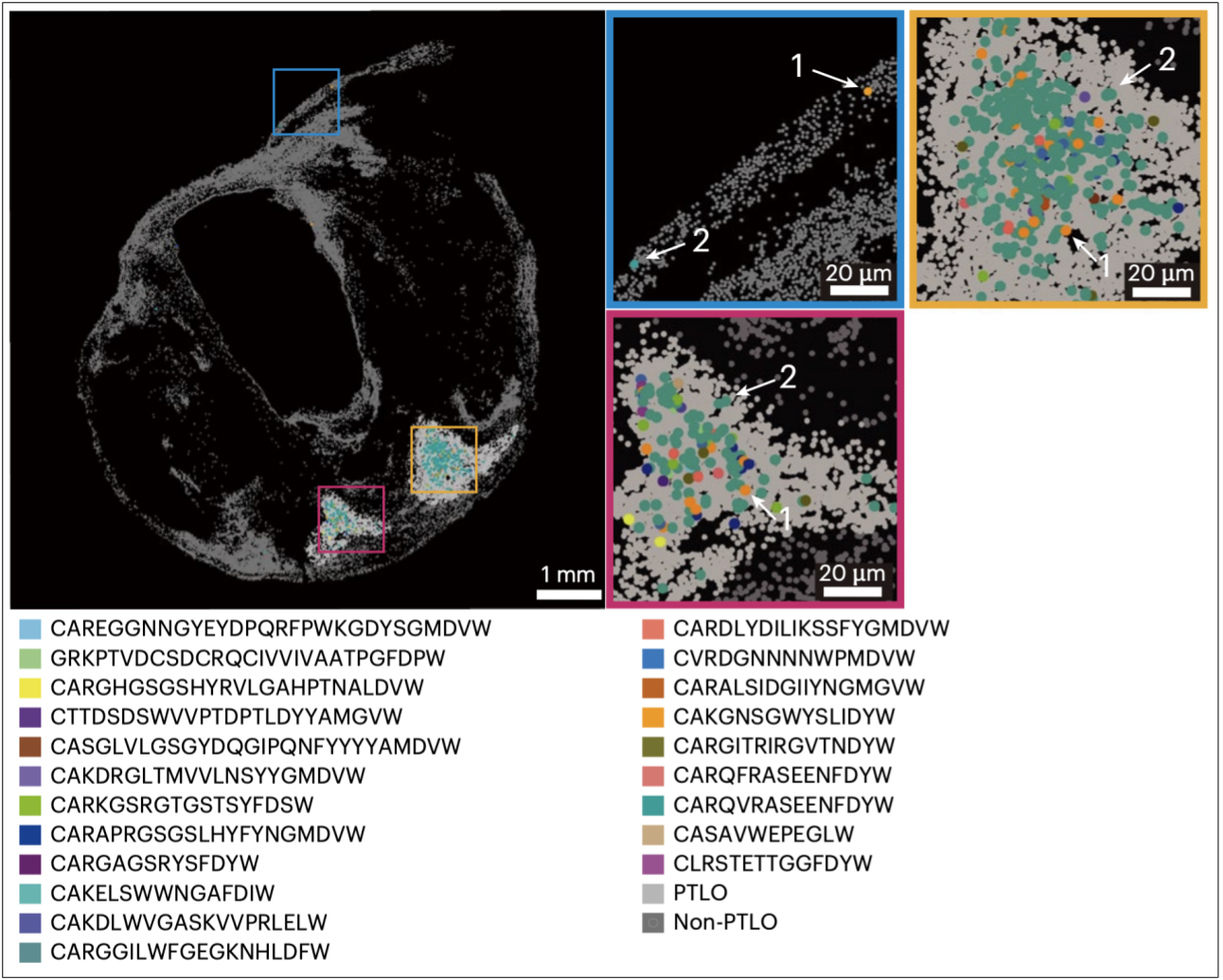 Expanded B‑cell clones (coloured dots) cluster inside PTLOs and taper outwards, linking antibody maturation to plaque instability.
Expanded B‑cell clones (coloured dots) cluster inside PTLOs and taper outwards, linking antibody maturation to plaque instability.
Real‑world samples underline the threat: among 149 carotid‑endarterectomy specimens, plaques containing PTLOs were an independent red flag for cerebrovascular events, with an adjusted odds ratio of 3.5 even after accounting for age, lipids and C‑reactive protein. Immunostaining revealed more IgG‑ and CD16‑double‑positive macrophages, cells linked to plaque rupture, in PTLO‑positive tissue than in PTLO‑negative plaques. The take‑home for clinicians and patients alike: spotting PTLOs could help identify high‑risk plaques early, while blocking their chemokine signals or IgG–FcγR crosstalk might keep dangerous lesions stable.
Beyond the immediate clinical insight, the study showcases how BGI's spatial‑omics toolkit can turn fibrotic, calcified tissue into actionable single‑cell data, offering a blueprint for decoding other hard‑to‑analyse diseases. All raw data are freely available in the Genome Sequence Archive (HRA006030) and China National GeneBank (STT0000052 / CNP0004894), and the full analysis workflow is on GitHub (BGI‑Human_plaque). By opening the dataset to the world, the authors hope to accelerate new diagnostics and therapies that could stop strokes before they start.
With the plaque atlas now part of growing open‑access resource, the team is partnering with clinicians and industry to test chemokine blockers and FcγR modulators in pre‑clinical models, aiming to disarm PTLOs before they can ignite the next vascular emergency. Eligible patients provided informed written consent before their enrollment in this study. All patients also consented to the publication of the data.
This study can be accessed here: https://www.nature.com/articles/s44161-025-00639-9



