BGI-Research, in collaboration with Lanzhou Veterinary Research Institute of the Chinese Academy of Agricultural Sciences, published their latest study in the Volume12, Issue18 of Advanced Science as frontispiece story. For the first time, the research team mapped the spatiotemporal dynamics of the liver tissue in mice infected with Echinococcus multilocularis (E. multilocularis) at high resolution using BGI's spatial transcriptomics technology, Stereo-seq. This study allowed scientists to track the immune response in situ, mapping how different immune cells, such as neutrophils, macrophages, and fibroblasts, interact during disease progression.
 The study “Spatiotemporal Transcriptomic Profiling Reveals the Dynamic Immunological Landscape of Alveolar Echinococcosis” was published in Advanced Science as frontispiece story.
The study “Spatiotemporal Transcriptomic Profiling Reveals the Dynamic Immunological Landscape of Alveolar Echinococcosis” was published in Advanced Science as frontispiece story.
 Wan-Zhong Jia, Junhua Li, Hong-Bin Yan, and co-workers decipher the shift in host (Nezha) immune response strategies from “active killing” to “negative segregation” during Echinococcus multilocularis hepatic infection. During the early infection stage, neutrophils (fire-tipped spear) and macrophages (wind fire wheels) collaborate to eliminate protoscoleces (goblins). In the middle and late stages, macrophages and fibroblasts (red armillary sash) cooperate to physically isolate the continuously expanding microcyst (monster). This research has been selected as the frontispiece story in Volume 12, Issue 18 of Advanced Science.(Design: Zhihua Ou, Jialing Chen, Peidi Ren, Hongbin Yan, Guoqiang Zhu; Painting: Tingting Ren)
Wan-Zhong Jia, Junhua Li, Hong-Bin Yan, and co-workers decipher the shift in host (Nezha) immune response strategies from “active killing” to “negative segregation” during Echinococcus multilocularis hepatic infection. During the early infection stage, neutrophils (fire-tipped spear) and macrophages (wind fire wheels) collaborate to eliminate protoscoleces (goblins). In the middle and late stages, macrophages and fibroblasts (red armillary sash) cooperate to physically isolate the continuously expanding microcyst (monster). This research has been selected as the frontispiece story in Volume 12, Issue 18 of Advanced Science.(Design: Zhihua Ou, Jialing Chen, Peidi Ren, Hongbin Yan, Guoqiang Zhu; Painting: Tingting Ren)
E. multilocularis is a zoonotic parasite responsible for alveolar echinococcosis (AE), which has been defined by the World Health Organization (WHO) roadmap 2021-2030 as one of the 20 targeted neglected diseases for control or elimination in impoverished areas. AE primarily occurs in the Northern Hemisphere, with a high incidence rate reported in the pastoral areas of Asia, North America and Europe.
Human become infected by eating food or drinking water contaminated by eggs of E. multilocularis. AE can lead to severe liver damage and failure, and the disease is highly lethal, with the untreated AE patients facing mortality rate exceeding 90%. Due to the difficulty of its early diagnosis and the complexity of its treatment - particularly compounded by immunosuppression occurring in late stages - AE is often fatal and it is difficult to cure.
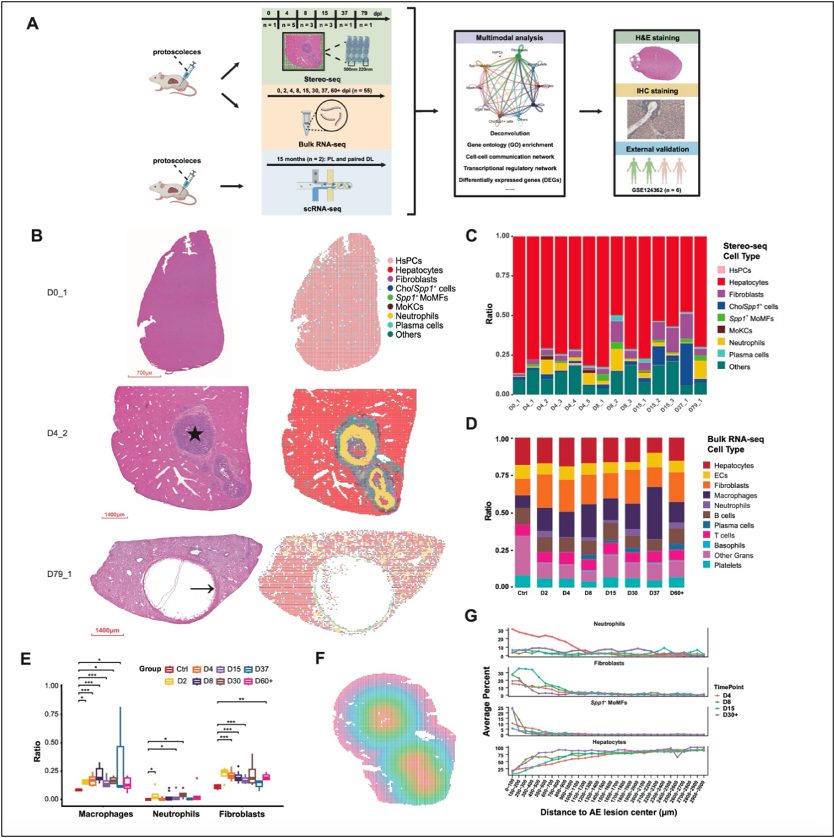 Spatiotemporal transcriptomic profiling of mouse liver infected with Echinococcus multilocularis.
Spatiotemporal transcriptomic profiling of mouse liver infected with Echinococcus multilocularis.
The research team generated a highly detailed map of immune cell infiltration around the infection sites and studied how these cells function during the different stages of AE. The study found two distinct types of neutrophils in the liver samples of E. multilocularis infected mice. The first type, marked by high IL-1β, was particularly active in the early infection stage, aggressively fighting the parasite by forming NETs (immune traps) and phagocytosing the invaders (E. multilocularis). These neutrophils were like front-line soldiers, working hard to eliminate the threat. The second type, with high levels of myeloperoxidase (MPO), was more scattered and less aggressive, suggesting a less intense immune response. These findings suggest that neutrophils are functionally heterogeneous during AE infection.
The study also highlighted the dual role of Spp1+ macrophages, which were mainly located around the infection sites. These macrophages exhibited both pro-inflammatory and anti-inflammatory phenotypes depending on the stage of the infection. Early in the infection, Spp1+ macrophages actively killed parasites and recruited additional immune cells. However, as the infection lingered and the parasites grew, these macrophages switched strategies, promoting tissue scarring (fibrosis) and helping the parasite evade the immune system. This transition from an "active killing" strategy to a "negative segregation" strategy is critical in understanding how the immune system adapts to chronic infections.
At later stages of infection, fibroblasts, which are cells involved in tissue repair, also play a role by increasing in number and forming fibrous tissue around the parasites. While this fibrosis trapped the parasites, it also hindered immune cells from effectively targeting them. The immune response thus shifted from aggressive parasite-killing to containing the infection, providing insight into potential treatments for chronic stages of AE.
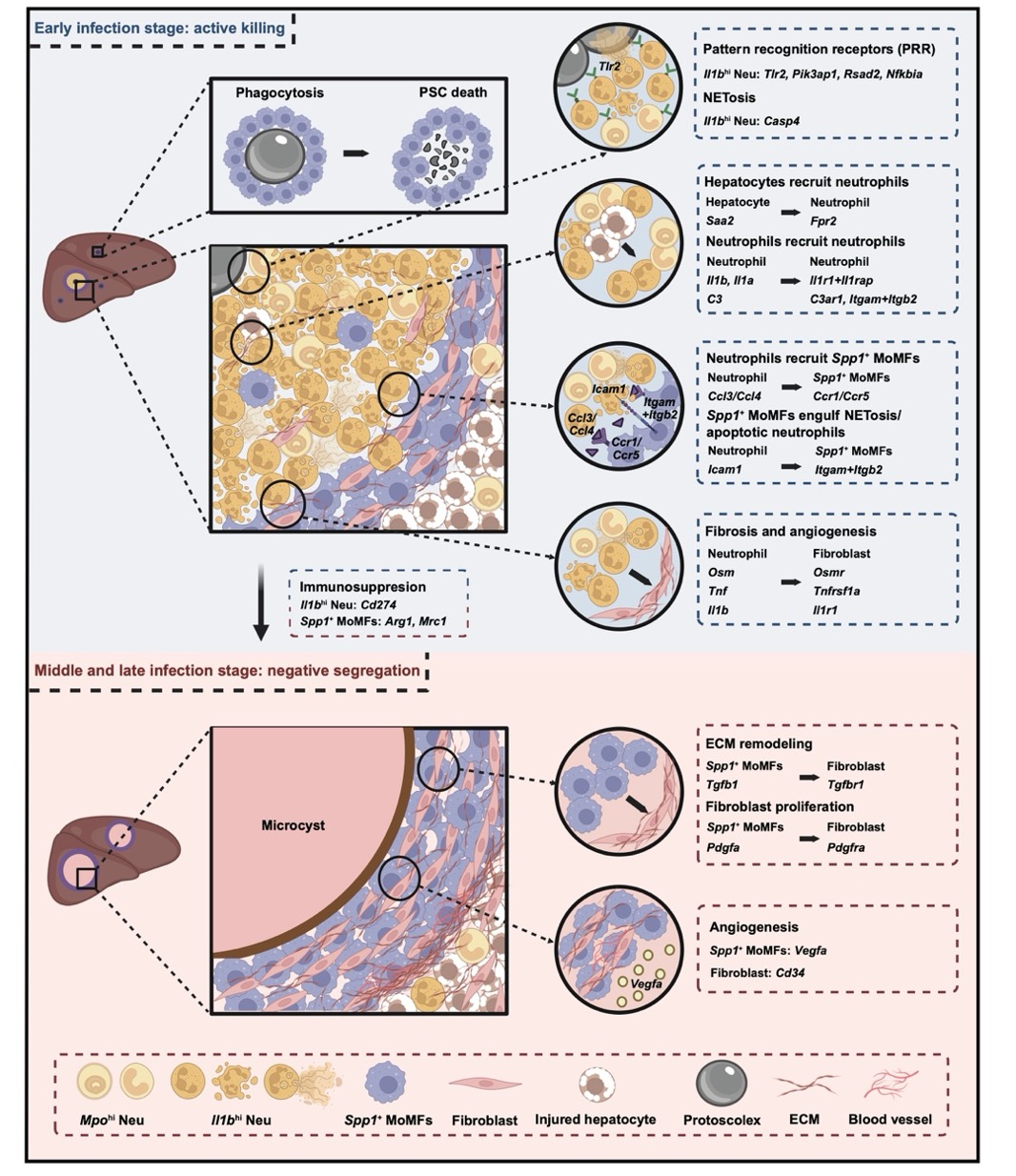 Schematic graph showing the functional involvements of neutrophils, macrophages, and fibroblasts during AE progression.
Schematic graph showing the functional involvements of neutrophils, macrophages, and fibroblasts during AE progression.
This finding is pivotal for understanding the pathogenesis of AE and offers potential therapeutic targets, such as modulating macrophage function or reversing fibrosis, to improve patient outcomes in the later stages of the disease.
Ethical approval for this study was obtained.
This research can be accessed here: https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202405914



