For the estimated 416 million people worldwide on the Alzheimer's disease continuum, including 316 million in preclinical stages, current treatment options remain frustratingly limited. While decades of research have focused on clearing amyloid beta protein deposits from the brain, this approach has yielded few effective therapies.
Now, scientists at BGI-Research and collaborators have uncovered an unexpected ally: beneficial bacteria living in our intestines that produce a powerful anti-inflammatory compound called indole-3-lactic acid (ILA). Published in Alzheimer's & Dementia, the team presents compelling evidence that the ability of gut bacteria to produce ILA, which becomes significantly impaired in people at the earliest stages of Alzheimer's disease, potentially removing crucial brain protection. More importantly, they demonstrate that this deficit can be corrected using a precisely combination of beneficial bacteria and prebiotics, leading to dramatic improvements in memory and brain health in laboratory studies.
 BGI-Research and Northwest A&F University report in Alzheimer’s & Dementia that the metabolite indole-3-lactic acid drived from gut bacteria is a key brain anti-inflammatory signal, and a targeted synbiotic can restore it - opening a new avenue for Alzheimer’s prevention and therapy.
BGI-Research and Northwest A&F University report in Alzheimer’s & Dementia that the metabolite indole-3-lactic acid drived from gut bacteria is a key brain anti-inflammatory signal, and a targeted synbiotic can restore it - opening a new avenue for Alzheimer’s prevention and therapy.
The discovery began when researchers analyzed intestinal bacteria samples from 164 individuals, comparing those with mild cognitive impairment to healthy controls using BGI-Research's computational analysis tools and the Expanded Cultivated Genome Reference (CGR2) database previously constructed. This revealed that two key bacterial enzymes, IL4I and fldH, which convert dietary tryptophan into the protective compound ILA, showed markedly reduced activity in people showing early cognitive decline.
"Think of it like an encrypted telegraph line from the gut to the brain that has been severed," explains Dr. Yuanqiang Zou, co-corresponding author and researcher at BGI-Research. "The decline in ILA levels may cause the brain to lose a crucial layer of anti-inflammatory protection."
Validation studies in mouse models confirmed the same pattern: ILA concentrations in both intestinal samples and blood were significantly lower in affected animals, while brain inflammation and amyloid deposits increased correspondingly.
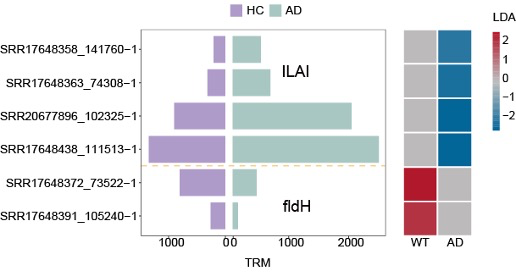 Sequencing of 164 human stool samples shows IL4I and fldH - two key ILA-biosynthetic genes - are markedly less abundant in preclinical AD, signalling a breakdown in gut-to-brain anti-inflammatory signalling.
Sequencing of 164 human stool samples shows IL4I and fldH - two key ILA-biosynthetic genes - are markedly less abundant in preclinical AD, signalling a breakdown in gut-to-brain anti-inflammatory signalling.
Rather than simply documenting this problem, researchers designed a solution by systematically screening CGR2, containing genetic blueprints for 3,324 bacterial strains. Through careful analysis of the ILA production pathway, they identified 23 candidate strains with ILA-manufacturing capabilities. High-performance liquid chromatography testing revealed Lactobacillus suilingensis as the clear winner, producing ILA at approximately 5.6 μg/mL, significantly outperforming other candidates. Growth experiments demonstrated that inulin, a fiber naturally found in foods like onions and garlic, served as the ideal fuel source at 20 g/L concentration. When combined, this synbiotic pairing produced substantially more ILA than either component alone, creating a high-performance biological system for restoring the missing gut-brain protection signal.
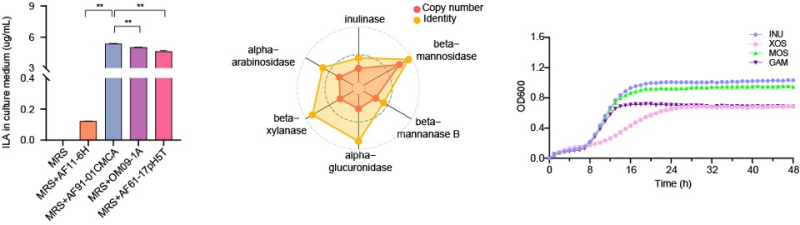 Screening pinpoints Lactobacillus suilingensis as the top ILA-producing strain and shows that inulin best supports its growth and metabolite yield.
Screening pinpoints Lactobacillus suilingensis as the top ILA-producing strain and shows that inulin best supports its growth and metabolite yield.
The therapeutic potential became clear when the team administered their optimized synbiotic to 7-month-old female mice with Alzheimer's-like symptoms. After just four weeks of treatment, animals demonstrated substantial improvements in spatial memory performance during Barnes maze testing, approaching healthy control levels. Brain tissue analysis revealed the biological basis: animals receiving synbiotic treatment showed significantly fewer amyloid plaques alongside marked reduction in activated microglia, the brain's inflammatory cells. Correspondingly, inflammatory molecules TNF-α, IL-1β, and COX-2 decreased substantially, while brain-derived neurotrophic factor (BDNF) increased significantly.
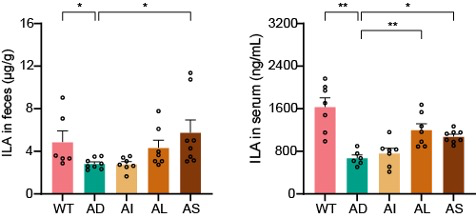 Synbiotic treatment significantly raises indole-3-lactic acid levels in both faeces and serum, restoring the gut-to-brain anti-inflammatory signal.
Synbiotic treatment significantly raises indole-3-lactic acid levels in both faeces and serum, restoring the gut-to-brain anti-inflammatory signal.
Mechanistic studies revealed that elevated ILA activates the aryl hydrocarbon receptor (AhR), a molecular switch regulating inflammatory responses throughout the body. In brain tissue, AhR activation triggers anti-inflammatory gene expression while suppressing the NF-κB signaling pathway that drives neuroinflammation. When researchers blocked AhR signaling with a specific inhibitor, the protective effects disappeared, definitively establishing the ILA-AhR pathway as the critical therapeutic mechanism.
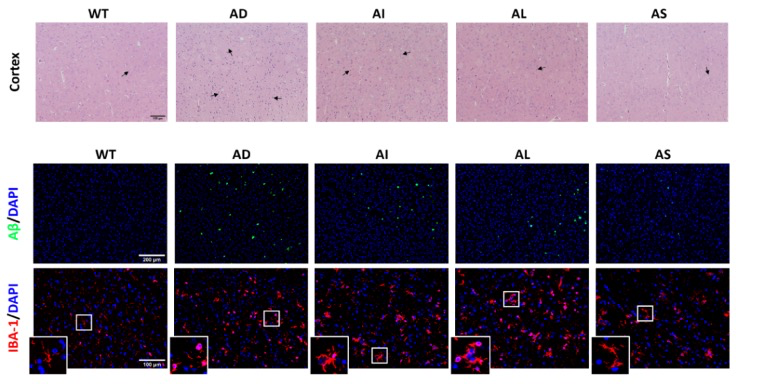 Combined H&E and dual-label immunofluorescence of cortical sections reveal preserved neuronal morphology plus a sharp drop in amyloid-β plaques and Iba-1-positive microglial activation after synbiotic therapy, underscoring its neuroprotective, anti-amyloid and anti-inflammatory potency.
Combined H&E and dual-label immunofluorescence of cortical sections reveal preserved neuronal morphology plus a sharp drop in amyloid-β plaques and Iba-1-positive microglial activation after synbiotic therapy, underscoring its neuroprotective, anti-amyloid and anti-inflammatory potency.
This breakthrough illuminates multiple clinical applications: ILA levels could serve as an early diagnostic marker for preclinical Alzheimer's disease, enabling intervention before irreversible brain damage occurs. The study provides proof-of-concept for the first targeted synbiotic formulation designed specifically to restore protective tryptophan metabolism in neurodegenerative disease. Because both components are derived from natural food sources, this approach could potentially be developed into a preventive strategy for at-risk populations.
As researchers continue unraveling complex gut-brain connections, this scientific revolution beginning in the intestinal tract has potential to transform prevention and intervention strategies for cognitive aging. Further investigations in diverse animal models and human volunteers will be needed to determine safety, optimal dosing and long-term efficacy before this microbiome-guided strategy can advance to clinical trials.
All human microbiome data were obtained from a previously published cohort with informed consent, and animal protocols were approved by the Animal Ethics Committee of Northwest A&F University following the Guide for Care and Use of Laboratory Animals (Eighth Edition).
This research can be assessed here: https://doi.org/10.1002/alz.70406



