On September 26, in a groundbreaking study published in Science, comprehensive single-cell spatial transcriptomic atlases of the cerebellar cortex across multiple species has been unveiled, offering new insights into the complexity and evolutionary adaptations of this critical brain region. This achievement was collaboratively completed by a team of nearly 100 researchers from the BGI-Research, Institute of Neuroscience of the Chinese Academy of Sciences, Kunming Institute of Zoology of the Chinese Academy of Sciences, South China University of Technology, Lingang Laboratory, Tencent AI Lab, and other institutions.
 The Study “Cross-species single-cell spatial transcriptomic atlases of the cerebellar cortex” is published in Science.
The Study “Cross-species single-cell spatial transcriptomic atlases of the cerebellar cortex” is published in Science.
The cerebellum's basic structure is deceivingly simple, comprising uniform cell types and circuitry that conceal its functional diversity. This recent study, however, reveals that beneath this uniformity lies a vast molecular diversity, particularly when comparing species such as mice, marmosets, and macaques. Such comparisons are vital because, unlike mice, the primate cerebellum has expanded significantly throughout evolution, resulting in increased neuron numbers and more sophisticated cognitive abilities.
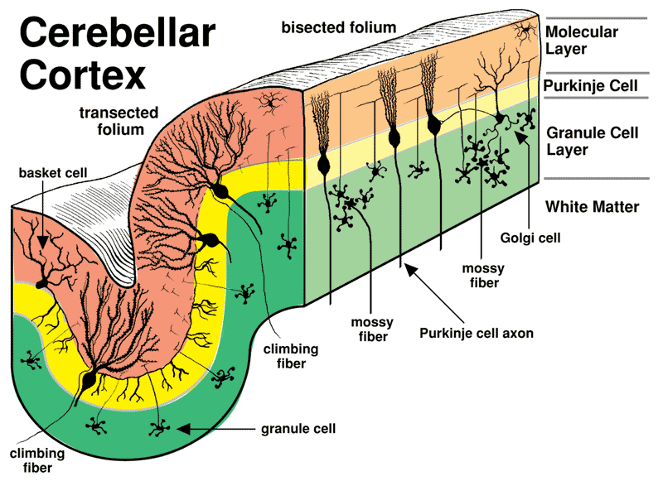 Neuronal composition of the cerebellar cortex. (Curtesy of College of Veterinary Medicine, University of Minnesota)
Neuronal composition of the cerebellar cortex. (Curtesy of College of Veterinary Medicine, University of Minnesota)
This research was made possible by the use of a cutting-edge spatial sequencing technology known as Stereo-seq. Developed by BGI-Research, this method allows for the mapping of gene expression across the entire cerebellum with a high resolution of about 500 nanometers. Such detail enables scientists to observe the exact locations of different cell types, and their gene expression patterns, providing a clearer picture of how the cerebellum’s cellular landscape contributes to its overall function.
Creating such a map is of great importance. By analyzing the gene expression patterns within the atlases, scientists can understand how various cell types interact and coordinate to perform the cerebellum's diverse functions. Additionally, the atlases can aid in identifying potential disease mechanisms, as diseases often trigger abnormal gene expression changes, which can be detected through the atlases.
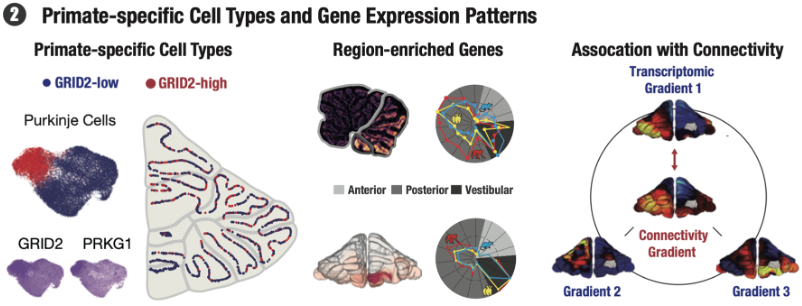
One of the most striking findings of the research is the discovery of primate-specific subtypes of Purkinje cells), which have not been observed in mice which was consolidated by the same Purkinje cell subtypes and similar gene expression signatures in previous human Purkinje single-cell sequencing data as in the two primates. These cells play pivotal roles in the cerebellum’s function, yet their unique properties in primates hint at evolutionary modifications that could underpin complex behaviors and cognitive processes unique to primates.
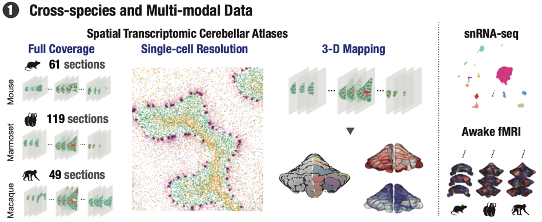 Cross-species multimodal atlas of the cerebellum.
Cross-species multimodal atlas of the cerebellum.
Further enriching these findings, the study utilized resting-state functional magnetic resonance imaging (fMRI) data from awake animals, including mice and primates. This data revealed functional connectivity gradients within the cerebellar cortex—patterns of how different areas of the cerebellum are interconnected and interact. Interestingly, these connectivity patterns correlate strongly with the spatial distribution of gene expression identified through Stereo-seq, suggesting a direct link between the cerebellum’s genetic makeup and its functional roles.
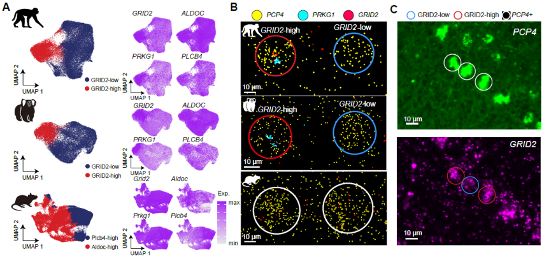 Primate-specific subtypes of Purkinje neurons.
Primate-specific subtypes of Purkinje neurons.
 Key findings of this study.
Key findings of this study.
Moreover, the identification of specific genes that vary between species and correlate with functional connectivity provides crucial insights into how different species have adapted their cerebellar functions over millions of years of evolution. Such knowledge not only enhances our understanding of the brain’s evolutionary trajectory but also opens up potential new avenues for investigating neurological diseases that may affect these very structures.
Overall, this comprehensive study provides an invaluable resource for further research into the cerebellum’s role in health and disease and illustrates the power of modern genomic technologies to uncover the hidden complexities of the brain. By detailing the specific cellular and molecular differences across species, the study not only sheds light on the evolutionary history of the cerebellum but also sets the stage for future investigations that could translate these findings into therapeutic strategies for cerebellar-related disorders.
Ethical review approval was obtained for this study.
The study can be accessed here: https://www.science.org/doi/10.1126/science.ado3927



