Salmonellosis, tuberculosis, whooping cough (pertussis), and sexually transmitted infections such as chlamydia and gonorrhea, are common infectious diseases caused by bacteria. Over the centuries, they have claimed countless lives.
The world has long struggled to find a cure for these bacterial infections. In his recently published book ‘The Good Virus: The Amazing Story and Forgotten Promise of the Phage,’ science journalist Tom Ireland explores the remarkable potential of bacteriophages (phages), viruses that attack bacteria, as a powerful disease-fighting approach.
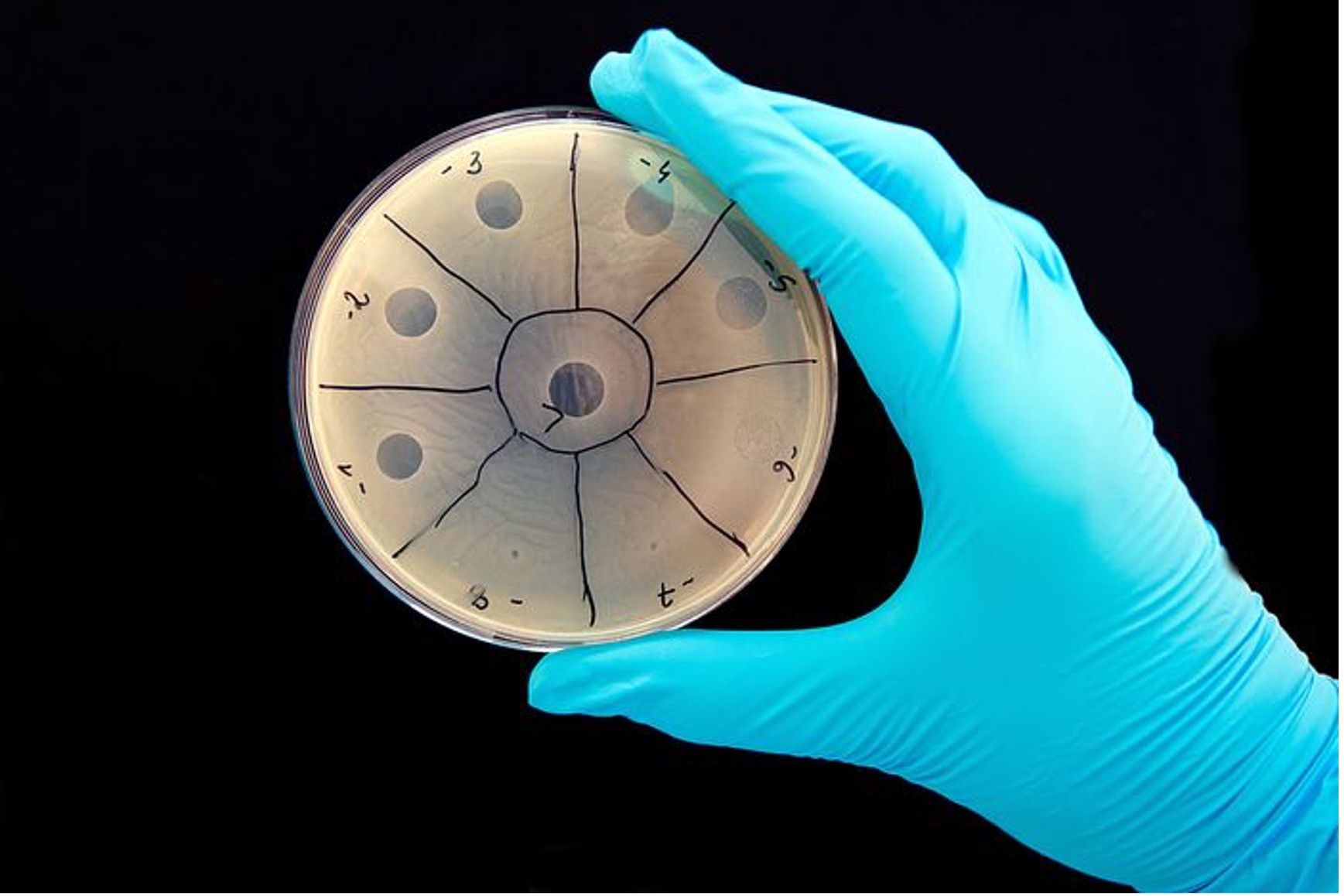 A petri dish of bacteria. The clear spots are areas where phage have killed the bacterium.
A petri dish of bacteria. The clear spots are areas where phage have killed the bacterium.
(PHOTO: BORZYWOJ/ALAMY; SOURCE: THE WALL STREET JOURNAL)
Bacteriophages are abundant and ubiquitous in nature, found everywhere, from the human body to frigid mountain elevations. These tiny viruses have a unique structure, with a protein capsid containing the genetic material, and they act like tiny bulb syringes to inject their DNA into bacteria. Once inside, they hijack the bacterial machinery to replicate and release new virus particles, ultimately leading to the destruction of the bacterium.
Phage therapy, once considered outdated, is now experiencing a renaissance. With the rise of antibiotic resistance, there is renewed interest in phages as a potential solution.
After years of being overshadowed, phage therapy may finally come to the forefront of modern medicine as a vital weapon against bacterial infections. Researchers and biotechnologists are actively working on synthesizing pharmaceutical-grade phages and streamlining the process of isolating phages from bacteria, in order to seek regulatory approval for phage-based medicines.
To forge such a weapon, scientists have investigated various ways to engineer and create suitable phages for specific bacteria.
In 2019, Rebekah M. Dedrick and her team at the University of Pittsburgh used engineered bacteriophages to alleviate the symptoms of a 15-year-old patient with cystic fibrosis with a disseminated Mycobacterium abscessus infection. The work is the first to demonstrate the safe and effective use of engineered bacteriophages in a human patient.
Last year, Jerry A. Nick and his team at the University of Colorado successfully used engineered bacteriophages to cure a drug-resistant Mycobacterium abscessus infection in a young patient awaiting a lung transplant with severe cystic fibrosis lung disease.
The above examples have demonstrated the unique value of bacteriophages in treating diseases, calling for further investigations by the global science community. The designer phage was also included in the World Economic Forum's Top 10 Emerging Technologies of 2023 report, which lists new technologies poised to impact the world in the next three to five years.
However, the process of engineering bacteriophages often faces multiple challenges, involving multiple hosts and platforms, which are not only time-consuming and labor-intensive but also highly expensive.
To address this, BGI-Research has led the development of a new "One-Pot” method, or “Stepping-stone Host Assisted Phage Engineering (SHAPE)” strategy, a bacteriophage genome engineering platform. This innovative approach uses model strains, such as Escherichia coli (E. coli), as stepping-stone hosts to combine the procedures of genome assembly, editing, and rebooting together, allowing the rebooting of bacteriophage genomes of up to 156 Kb in a single step. This study has been published in Cell Report Methods.
 The research “Harnessing stepping-stone hosts to engineer, select, and reboot synthetic bacteriophages in one pot” published in Cell Report Methods.
The research “Harnessing stepping-stone hosts to engineer, select, and reboot synthetic bacteriophages in one pot” published in Cell Report Methods.
The study has demonstrated the platform's broad coverage of engineering by successfully constructing a first generation of stepping-stone host, along with cross-genus and cross-order rebooting of 90 phages, capable of targeting four clinically significant pathogenic bacteria, including Klebsiella pneumoniae, Salmonella enterica, Pseudomonas aeruginosa, and Acinetobacter baumannii.
This strategy not only reduces biological safety risks but also enables easy and cost-effective genome assembly, editing, and rebooting, significantly enhancing the output efficiency of engineered bacteriophages.
With the "One-Pot” method platform, a more comprehensive range of bacteriophages can be covered, contributing to the fight against multidrug-resistant bacteria and benefiting both patients suffering from bacterial infections and bacteriophage researchers.
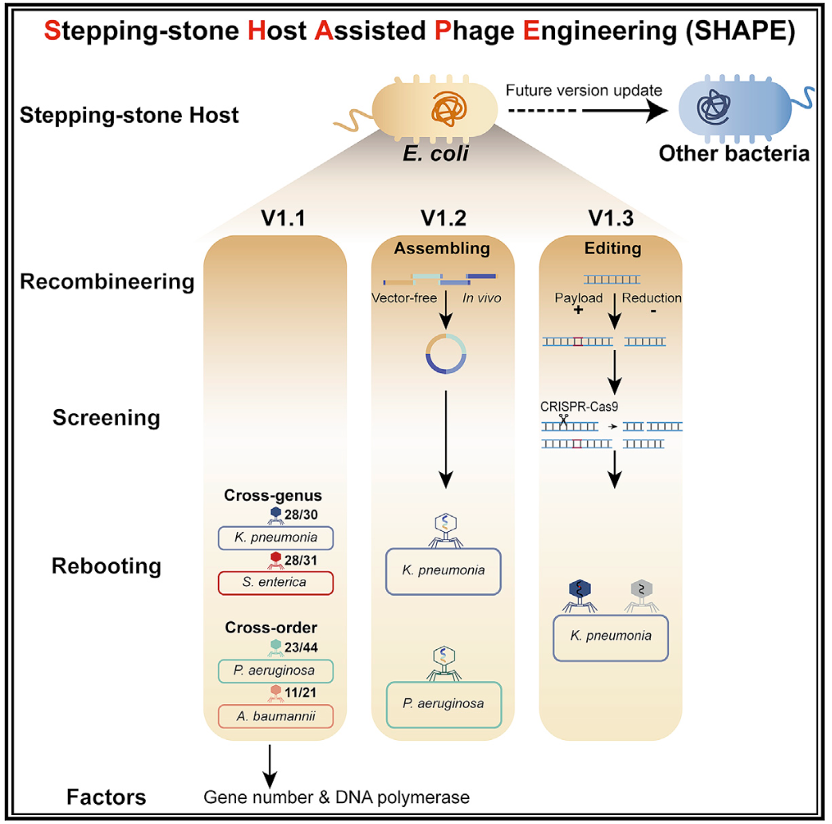 Working principle of the "One-Pot” method, or “Stepping-stone Host Assisted Phage Engineering (SHAPE)” strategy.
Working principle of the "One-Pot” method, or “Stepping-stone Host Assisted Phage Engineering (SHAPE)” strategy.
In addition, BGI-Research has established a large-scale Global Phage Hub (GPH). It integrated bacteriophage isolates, genomic sequences, and phenotypic datasets, to explore the phenomena, patterns, and mechanisms of bacteriophages. The team has developed a series of data mining tools and a research platform that combines artificial intelligence and synthetic biology.
The research team is also working with medical institutions to develop bacteriophage therapy for bacterial infections and chronic diseases.
In the future, these tiny but powerful creatures might be the solution to tackling the challenges in global public health.



