When a new lung is sewn into a patient, the first 72 hours are a knife edge: approximately 15% to 25% of recipients develop severe primary graft dysfunction (PGD), an explosive form of sterile inflammation that floods the graft with fluid and starves it of oxygen, representing the principal cause of short-term mortality following lung transplantation.
In a cover story in Cell Reports Medicine, researchers at Shanghai Pulmonary Hospital, working with BGI-Research and partners, trace that storm to a rare, metabolically overcharged subset of white blood cells: CD177⁺ neutrophils. They show that blocking their mitochondrial complex I rescues lungs in two transplant models while simultaneously providing clinicians with an early blood-based biomarker for the sickest patients.
 The cover of Cell Reports Medicine Volume 6, Issue 5. A ring-shaped "island" evokes the lung transplant recipient; semi-submerged aquatic plants depict the injured graft, while the surrounding greenery embodies infiltrating CD177⁺ neutrophils. The bright blossoms at the island's center symbolize their overactive mitochondrial complex I, and the invasive vines represent neutrophil extracellular traps (NETs) eroding lung tissue. Parasitic dodder entwines the vines, signifying how the complex I inhibitor IACS-010759 halts NET formation and preserves graft integrity.
The cover of Cell Reports Medicine Volume 6, Issue 5. A ring-shaped "island" evokes the lung transplant recipient; semi-submerged aquatic plants depict the injured graft, while the surrounding greenery embodies infiltrating CD177⁺ neutrophils. The bright blossoms at the island's center symbolize their overactive mitochondrial complex I, and the invasive vines represent neutrophil extracellular traps (NETs) eroding lung tissue. Parasitic dodder entwines the vines, signifying how the complex I inhibitor IACS-010759 halts NET formation and preserves graft integrity.
 The study “Targeting mitochondrial complex I of CD177+ neutrophils alleviates lung ischemia-reperfusion injury” was published in Cell Reports Medicine as a cover story.
The study “Targeting mitochondrial complex I of CD177+ neutrophils alleviates lung ischemia-reperfusion injury” was published in Cell Reports Medicine as a cover story.
Leveraging BGI's high-throughput single-cell sequencing platform alongside the ultra-high-resolution Stereo-seq spatial transcriptomics technology, the team built a cell-by-cell and pixel-by-pixel atlas of mouse lungs subjected to ischemia reperfusion injury, the bedrock model of PGD. That atlas revealed a dramatic enrichment of CD177⁺ neutrophils in the precise zones of tissue damage, and multi-omics profiling showed why: these cells ramp up mitochondrial complex I activity, spewing reactive oxygen species and weaving neutrophil extracellular traps that shred the delicate air exchange surface. Conditional deletion of the Cd177 gene in mouse neutrophils, confirmed by flow cytometry and single-cell RNA sequencing, largely abolished oxidative burst, neutrophil extracellular trap (NET) formation and histological injury, demonstrating that CD177⁺ cells are key contributors to lung ischemia reperfusion injury.
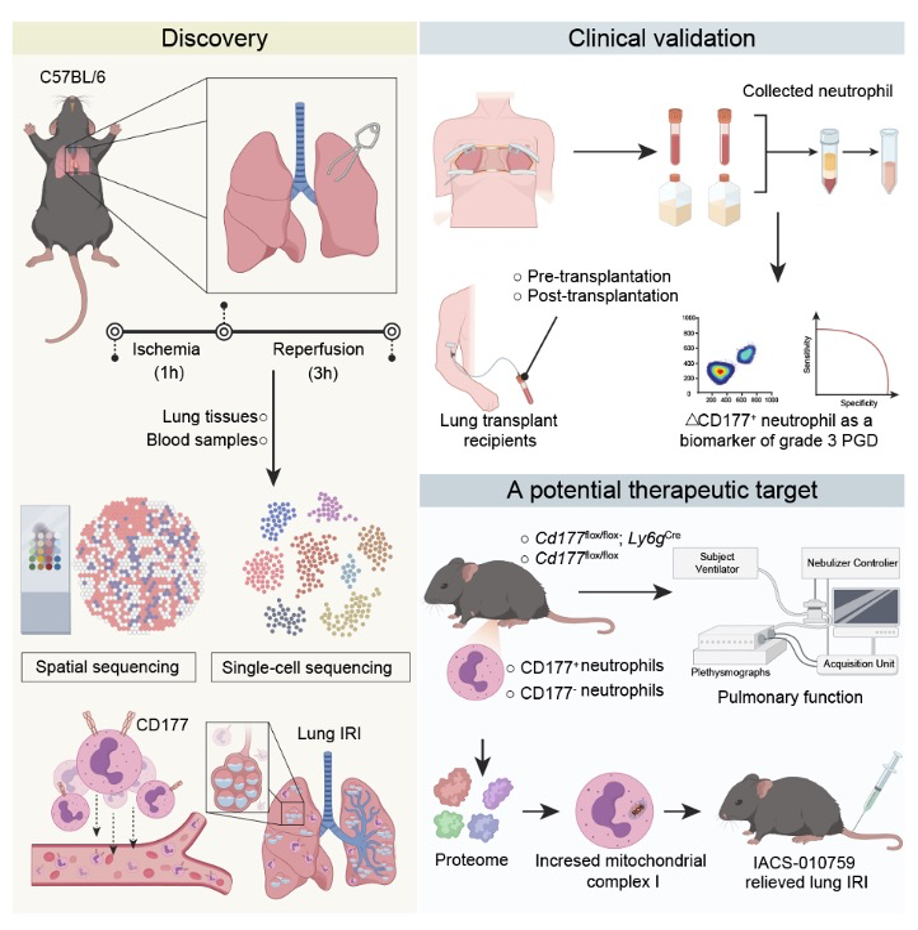 Single-cell and spatial sequencing of mouse ischemia reperfusion lungs pinpointed CD177⁺ neutrophils; a 104-patient cohort validated ΔCD177⁺ blood counts as an early biomarker for grade 3 PGD; complex I inhibition with IACS-010759 reduced NETs and restored lung function in mouse and rat transplant models.
Single-cell and spatial sequencing of mouse ischemia reperfusion lungs pinpointed CD177⁺ neutrophils; a 104-patient cohort validated ΔCD177⁺ blood counts as an early biomarker for grade 3 PGD; complex I inhibition with IACS-010759 reduced NETs and restored lung function in mouse and rat transplant models.
To probe clinical relevance, the investigators turned to a prospective cohort of 104 lung transplant recipients. By measuring the change in the proportion of CD177⁺ neutrophils in peripheral blood four hours after reperfusion (ΔCD177⁺), they could forecast grade 3 PGD with an area under the curve of 0.871, easily outperforming the conventional neutrophil to lymphocyte ratio. That four-hour window, the authors note, coincides with the moment intensivists decide whether to escalate oxygen, ventilation or extracorporeal support, making ΔCD177⁺ a timely triage tool.
Having identified the culprit and a diagnostic handle, the group asked whether the same cells could be therapeutically targeted. They chose IACS-010759, a highly selective inhibitor of mitochondrial complex I already in early-phase oncology trials. A single intravenous dose (1 mg/kg) delivered three hours before reperfusion cut oxygen consumption rates in CD177⁺ neutrophils, blunted NET production and, critically, reduced lung edema, improved compliance and raised arterial oxygen in both the classic mouse hilar-clamp model and an 18-hour cold ischemia orthotopic rat transplant. Histology corroborated the functional data: fewer infiltrating neutrophils, fewer citrullinated histone positive nets, intact alveolar walls.
The research demonstrates that targeting CD177⁺ neutrophils through mitochondrial complex I inhibition significantly relieved lung injury caused by ischemia reperfusion, while the diagnostic accuracy of ΔCD177⁺ neutrophils proved superior to conventional clinical factors. The findings suggest that both rapid CD177 testing and targeted metabolic interventions could provide new approaches for early detection and treatment of primary graft dysfunction in lung transplant patients.
Future work will push the blood assay into multicenter trials and explore antibody drug conjugates that ferry complex I inhibitors directly to CD177⁺ cells, trimming systemic toxicity observed in oncology studies. For now, the team has provided clinicians with both an early diagnostic tool to identify PGD and a therapeutic approach to prevent it: two long-sought advances in the quest to make lung transplantation a durable cure rather than a race against time.
The study offers a blueprint for tackling sterile inflammatory injury well beyond transplantation, as the single-cell sequencing platform generated most of the single-cell data, including from challenging formaldehyde-fixed transplant tissue, while Stereo-seq located the culpable cells with subcellular precision inside intact lung slices. The authors have deposited raw sequencing and proteomics files at CNGBdb and OMix, inviting the community to mine the dataset for additional therapeutic angles.
Ethical approval and written informed consent from the patients were obtained for this study.
This research can be assessed here: https://doi.org/10.1016/j.xcrm.2025.102140



