Early mammalian development unfolds in millimetre-sized embryos within hours, yet errors during this brief window cause nearly a third of all birth defects, from congenital heart disease to neural tube malformations. Using BGI's high-resolution Stereo-seq spatial transcriptomics, an international team led by Southeast University (Nanjing) and BGI-Research has reconstructed this critical developmental stage cell by cell in three dimensions. Their study, published 18 June in Cell, presents the world's first single-cell-precision, whole-embryo digital atlas of the mouse from late gastrulation (E7.5) through early organogenesis (E8.0) and identifies a Primordium Determination Zone (PDZ) at the embryonic/extra-embryonic boundary where heart and gut organ primor-dia originate.
 International collaboration led by Southeast University and BGI-Research publishes breakthrough digital embryo research in Cell, advancing our understanding of early organ development and birth defects.
International collaboration led by Southeast University and BGI-Research publishes breakthrough digital embryo research in Cell, advancing our understanding of early organ development and birth defects.
Reconstructing Complete "3D Digital Embryos" at Single-Cell Resolution
During mammalian embryonic development, a critically vulnerable stage exists where approximately 30% of congenital defects—including congenital heart disease and neural tube defects—originate from spatiotemporal dysregulation in cell fate determi-nation or migration. This phase spans from late gastrulation to early organogenesis, during which mammalian embryos undergo exponential cellular expansion (from hun-dreds to tens of thousands of cells) and experience their first major morphological transformation.
Due to the miniature size (1–2 mm) and structural intricacy of embryos at this stage, precise mapping of spatial coordinates and gene expression profiles for individual cells is essential. To address this, the research team employed Stereo-seq technology to serially section and sequence six intact mouse embryos at three key develop-mental timepoints: E7.5, E7.75, and E8.0. This approach successfully cap-tured 104,343 high-quality cells and reconstructed a high-resolution 3D dynam-ic "digital embryo". The digital embryo exhibits clearly delineated germ layer struc-tures and accurately restores the morphology of key tissues, including the notochord, primitive streak, and node.
The digital embryo reveals the precise choreography of gut tube development. The team discovered that the primitive gut forms through a unique saddle-like invagination process, where anterior gut endoderm and paraxial endoderm undergo coordinated morphogenetic movements to establish the foregut tube. This represents the first de-tailed spatial reconstruction of mammalian gut tube formation at single-cell resolution, providing insights into how errors in this process might lead to gastrointestinal birth de-fects.
The classification of cell subtypes and 3D reconstruction are of great significance for understanding organ origin, with the early development of the heart serving as the most representative example. By precisely classifying and spatially reconstructing car-diomyocytes and cardiac progenitor cell subtypes at embryonic day 8.0 (E8.0), the re-search team used the Spateo 3D spatial algorithm to localize first heart field (FHF) cells and found that these cells exhibit a mixed distribution with anterior second heart field (aSHF) cells at the embryonic-extraembryonic boundary in the anterior embryo. This discovery provides critical evidence for understanding the differentiation and spa-tial arrangement of cell subtypes during early heart development, facilitating in-depth dissection of the early cellular behaviors and molecular mechanisms underlying car-diac primordium formation.
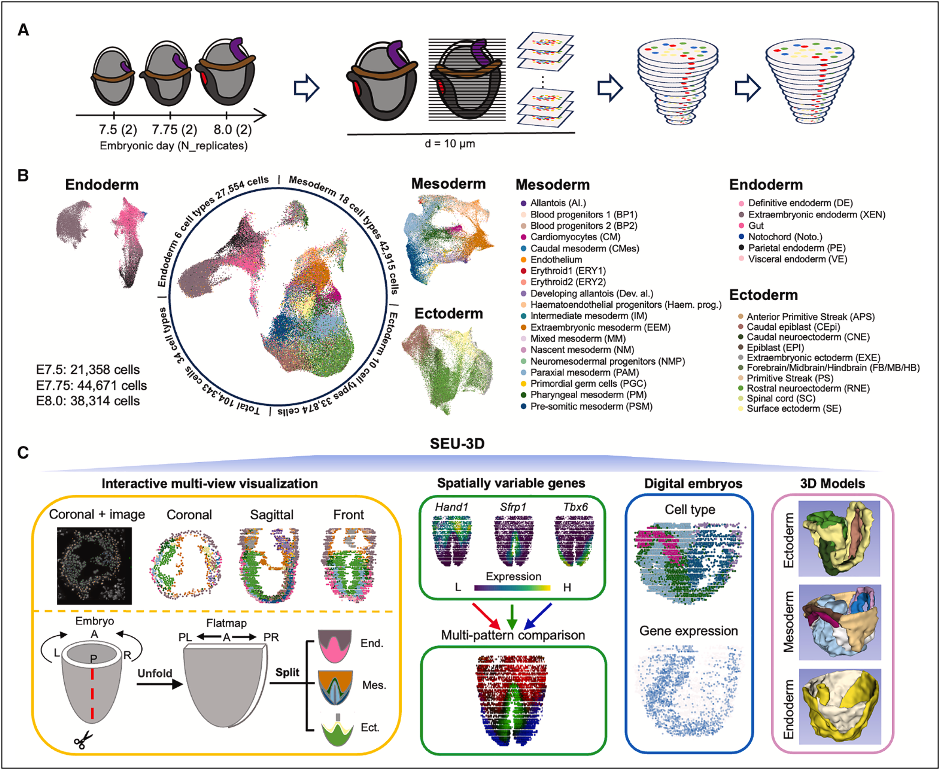 Experimental design, cell atlas of 34 types across three germ layers, and SEU-3D plat-form create the first complete digital embryo for developmental analysis.
Experimental design, cell atlas of 34 types across three germ layers, and SEU-3D plat-form create the first complete digital embryo for developmental analysis.
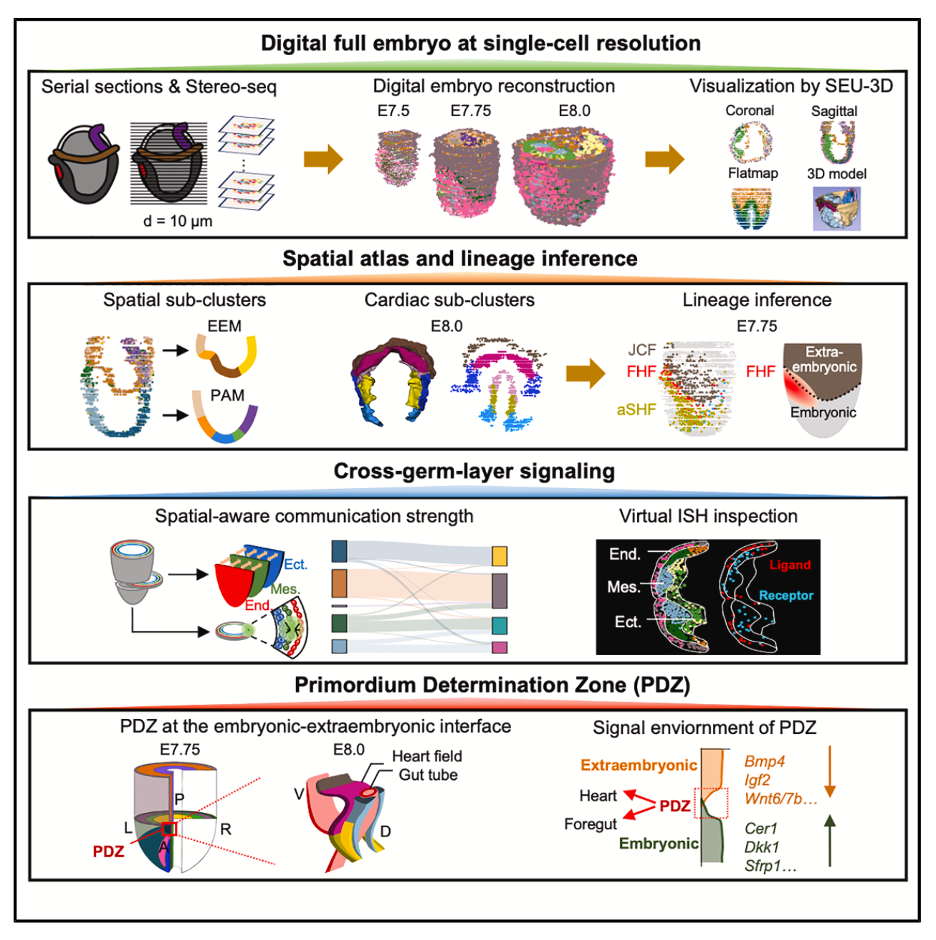 Study workflow from serial sectioning to 3D digital reconstruction reveals spatial and molecular events orchestrating early organ development.
Study workflow from serial sectioning to 3D digital reconstruction reveals spatial and molecular events orchestrating early organ development.
The Discovery of Organ Primordium Determination Zone
The research team leveraged Stereo-seq to identify a distinctive signaling-permissive niche adjacent to the embryonic-extraembryonic interface. This region, designated the primordium determination zone (PDZ), serves as a reservoir for cardiac and fore-gut organ primordia. Here, a unique signaling microenvironment emerges, character-ized by coordinated ligand-inhibitor gradients that drive synergistic development of heart and foregut structures. Critically, signaling pathways within the PDZ exert instructive roles in organ primordium specification; disruptions to these pathways predispose embryos to congenital malformations due to aberrant organogenesis.
"Thanks to the unique advantages of BGI's Stereo-seq 500nm high-resolution se-quencing technology, this study reconstructed the first complete, single-cell resolution 3D digital embryo, providing important insights into early organogenesis and offering data resources and technical methods for studying development and disease," said Dr. Xiaodong Fang, Vice President of BGI-Research and co-corresponding author. "Ste-reo-seq technology is now widely applied to 3D atlas construction of tissues and or-gans, helping us deepen our understanding of organ structure and function relation-ships."
The team anticipates that this digital foundation will accelerate investigations into de-velopmental mechanisms, disease origins and regenerative medicine approaches.
The full article can be accessed at https://doi.org/10.1016/j.cell.2025.05.035.



